Methane (CH 4 ) is a greenhouse gas emitted by industry, agriculture, and waste systems. Methane is
Question:
Methane (CH4) is a greenhouse gas emitted by industry, agriculture, and waste systems. Methane is the second most prevalent greenhouse gas (after carbon dioxide). Methane plays an important role in climate change because it absorbs infrared radiation more efficiently than carbon dioxide. Methane is broken down in the atmosphere by ozone (O3), making its atmospheric lifetime shorter than that of carbon dioxide.![]()
A research group studied the rate of the reaction by which methane reacts with ozone; the data are shown in the following tables. Study the data and answer the questions that follow.![Initial Rate vs. Initial [CH4] 0.010 0.020 0.020 [03] 0.010 0.010 0.020 Concentrations Initial Rate (M/s)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/5/774655714ceb5f7b1700205773653.jpg)
a. Use the data in the first table to determine the order of the reaction with respect to each reactant.
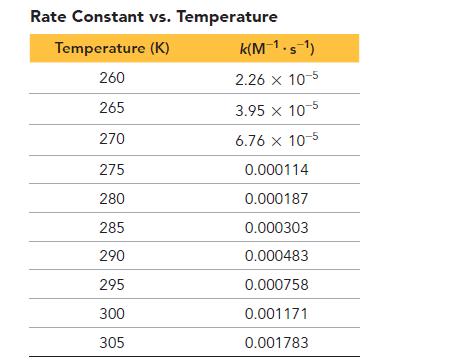
b. Use the data in the second table to determine the activation barrier and pre-exponential factor for the reaction.
c. Atmospheric concentrations of methane and ozone can vary depending on the location and altitude. Calculate the rate of the reaction at 273 K for a methane concentration of 1.8 ppm (by volume) and an ozone concentration of 5.0 ppm (by volume).
Note that 1 ppm of CH4 by volume means 1 L CH4/106 L air. Assume STP (standard temperature and pressure) so that 1 mol gas occupies 22.4 L.
d. What is the half-life of methane in the atmosphere in years at 323 K?
Step by Step Answer:






