Name each disubstituted benzene. a. Br Br b. CH - CH3 CH - CH3 C. F
Question:
Name each disubstituted benzene.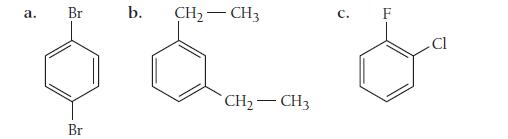
Transcribed Image Text:
a. Br Br b. CH₂ - CH3 CH₂ - CH3 C. F
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a 14dibromobenzene or pd...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Kekulé's time, cyclohexane was unknown, and there was no proof that benzene must be a six-membered ring. Determination of the structure relied largely on the known numbers of...
-
A method for determining the structures of disubstituted benzene derivatives was proposed in 1874 by Wilhelm Korner of the University of Milan. Kdrner had in hand three dibromobenzenes, A, B, and C,...
-
Consider the following information regarding corporate bonds: Rating AA A BB B CCC 0.5% 5.5% 12.2% 0.196 1.0% 0.2% 3.0% 2.2% 8.0% 3.5% 16.0% 48.0% Average Beta 0.05 0.05 0.10 0.17 0.26 0.31 Wyatt Oil...
-
Joan has been a full-time director of Sunnybank Pursuits Ltd (a trading company) since 2001 and has owned 10% of the company's ordinary shares since 2002. She retired in February 2021 and gave all of...
-
Albion Inc. provided the following information for its most recent year of operation. The tax rate is 40 percent. Sales ..................... $100,000 Cost of goods sold ................. $45,000 Net...
-
Produce an object-oriented database design for the DreamHome case study documented in Appendix A. State any assumptions necessary to support your design.
-
Procter and Gamble (P&G) and Unilever are the two leading firms in the consumer products industry for offerings such as soap, shampoo, and laundry detergent. P&G (www.pg.com) is based in the United...
-
McIver's Swimwear Distributors is a relatively small, privately held swimwear distribution company that operates in the Midwest and handles several product lines, including footwear, clothing, and...
-
A negative trend in Gross Margin may indicate that Cost of Sales is decreasing. True False
-
Name each disubstituted benzene. a. Br Cl b. CH - CH3 C.
-
Name each compound in which the benzene ring is best treated as a substituent. a. H3C-CH-CH-CH-CH-CH3 H3C b. CH,CH,CH,CH,C=CCH3 CH3 | c. CH3CH=CH-C=CHCHCH,CH3 T CH3 CH3
-
Consider a pistoncylinder assembly containing 0.10 kg of air initially at 300 K and 300 kPa (state 1). The piston moves such that 35.9 kJ of work is done on the air by the surroundings. The process...
-
Gulf Shore Lawn and Garden Maintenance provides two general outdoor services: lawn maintenance and garden maintenance. The company charges customers $18.0 per hour for each type of service, but lawn...
-
Two level sections of an east highway (G=0) are to be connected. Currently, the two sections of highway are seperated by a 4000-ft (horizontal distance), 2% grade. The westernmost section of highway...
-
A solution contains 2 x 10-3 moles Ca2+/L and 3 x 10-4 moles Mg2+/L. Given the formation constants for CaEDTA2- and MgEDTA2- of 1010.6 and 108.7, respecively, calculate: 1) Concentration of MgEDTA2-...
-
The direct material (DM) price variance is $2,650 favorable and the DM usage variance is $3,000 unfavorable. The budgeted amount of DM for each unit of product is 2 lbs. to be purchased at the...
-
On January 1, 2023, AMI Corporation purchased the non-cash net assets of Oriole Ltd. for $8,399,900. Following is the statement of financial position of Oriole Ltd. from the company's year- end the...
-
An individual leaves a college faculty, where she was earning $80,000 a year, to begin a new venture. She invests her savings of $20,000, which were earning 10 percent annually. She then spends...
-
The manager for retail customers, Katie White, wants to hear your opinion regarding one business offer she has received from an entrepreneur who is starting a mobile phone app called Easy Money. The...
-
A 2.2-nF capacitor and one of unknown capacitance are in parallel across a 10-V rms sine-wave generator. At 1.0 kHz, the generator supplies a total current of 3.4 mA rms. The generator frequency is...
-
Connections to the body for electrocardiography (ECG) and electroencephalography (EEG) are normally made with metal electrodes and conductive gels to ensure good electrical contact. An alternative is...
-
The FM radio band covers the frequency range 88108 MHz. If the variable capacitor in an FM receiver ranges from 10.9 pF to 16.4 pF, what inductor should be used to make an LC circuit whose resonant...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


