Nitrogen and hydrogen gas react to form ammonia according to the reaction: A flask contains a mixture
Question:
Nitrogen and hydrogen gas react to form ammonia according to the reaction:![]()
A flask contains a mixture of reactants represented by the image shown at the left.
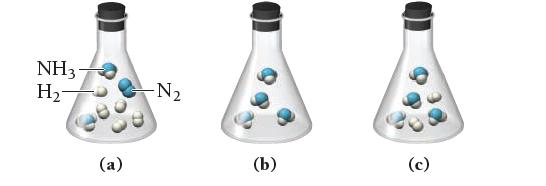
Which of the following images best represents the mixture in the flask after the reactants have reacted as completely as possible? What is the limiting reactant? Which reactant is in excess?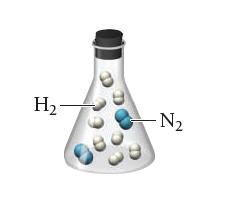
Transcribed Image Text:
N₂(g) + 3 H₂(g) →→→ 2 NH3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
c Nitrogen is the limiting reactant and t...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Nitrogen (N2) and hydrogen (H2) react to form ammonia (NH3). Consider the mixture of N2 and H2 shown in the accompanying diagram. The blue spheres represent N, and the white ones represent H. Draw a...
-
Nitrogen and hydrogen gases react to form ammonia gas as follows: N2 (g) + 3 H2 2NH3 (g) At a certain temperature and pressure, 1.2 L of N2 reacts with 3.6 L of H2. If all the N2 and H2 are...
-
Ammonia is created in the Haber process in a rigid container (nitrogen gas plus hydrogen gas react to form ammonia gas) at a constant temperature. 5 moles of hydrogen gas are mixed with 10 moles of...
-
We consider the following CFG SE + SIE E 01|2|3|4|5|67|8|9|(S) Apply the leftmost derivation and rightmost derivation with a top-down parser for this sentence: (3+7+ (1+4)) + 2
-
The financial statements of Hershey Foods are presented in Appendix B, following the financial statements for Tootsie Roll Industries in Appendix A. Instructions (a) Based on the information in these...
-
A typical new household refrigerator consumes about 680 kWh of electricity per year and has a coefficient of performance of 1.4. The amount of heat removed by this refrigerator from the refrigerated...
-
What is different about real option value (ROV) analysis compared to traditional net present value (NPV) analysis?
-
1. Based on Madelines Day 1 observations and conversation with Mr. Spencer, provide examples of the service quality gaps (e.g., knowledge, standards, delivery, communications, and service) that are...
-
Show steps how to get the amount of error ( Pduration - Pmarket ) for second question in the picture, the answer is - 0 . 7 5
-
The founder of Frenza asks us to assist her in accounting and analysis of the corporations bonds, which have an annual contract rate of 8%. She wants to know the business and accounting implications...
-
What is reaction stoichiometry? What is the significance of the coefficients in a balanced chemical equation?
-
Ammonia, NH 3 , can be synthesized by the reaction: Starting with 86.3 g NO and 25.6 g H 2 , find the theoretical yield of ammonia in grams. 2 NO(g) + 5 H(g) 2 NH3(g) + 2 HO(g)
-
2 kg of wet steam at \(10 \mathrm{bar}\) and \(90 \%\) dry is expanded according to the law \(p v=\) const. to a pressure of 1 bar. Determine the final condition of steam and the change in internal...
-
17. S T D -3 -2 graph of glx) 2 The graph of the continuous function g is shown above for -46x4. The Function g is twice differentiable, except at x=0. let & be the function with flo1=-2 and f'(x) =...
-
Tim works for HydroTech, a manufacturer of high-pressure industrial water pumps. He reports directly to the CFO, and she has asked him to calculate HydroTech's WACC. He has gathered the following...
-
You are appraising a 15,450 square foot (SF) building and using the Cost Approach. The base cost is $50/SF, the local multiplier is 1.05, the current cost multiplier is 0.92. The land value is...
-
How do you manage global and international teams? What would you do different?
-
Your friend is super excited about the results of their study! They examined whether different parenting styles [A] resulted in differences in anxiety levels among children. The different levels (a)...
-
What are the primary interests of researchers working in the following fields of genetics? A. Transmission genetics B. Molecular genetics C. Population genetics
-
A consumer magazine is evaluating five brands of trash compactors for their effectiveness in reducing the volume of typical household products that are discarded. In the experiment, each block...
-
The observed Boyle temperatures of H 2 , N 2 , and CH4 are 110, 327, and 510. K, respectively. Compare these values with those calculated for a van der Waals gas with the appropriate parameters.
-
For the Berthelot equation, V m = (RT/P) + b a (RT 2 ) find an expression for the Boyle temperature in terms of a, b, and R.
-
For a van der Waals gas, z = V m /(V m b) a/RTV m . Expand the first term of this expression in a Taylor series in the limit V m >> b to obtain z 1 + (b a/RT)(1/V m ).
-
A Planning Materiality Base of: Profit before tax A Planning Materiality percentage of: 5% A Planning Materiality amount of: $10,443,950 (5% x 208,879,000) A Clearly Trivial Threshold of : $261,100...
-
M-2 A particular cost is a mixed cost. This cost is $4,000 in total when 200 units are produced. Which of the following amounts is a possible value for this mixed cost if production increases to 250...
-
Under what grounds, no VAT shall be deducted or withheld at source?

Study smarter with the SolutionInn App


