Rank each set of substances in order of increasing standard molar entropy (S). Explain your reasoning. a.
Question:
Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning.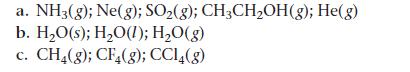
Transcribed Image Text:
a. NH3(g); Ne(g); SO₂(g); CH3CH₂OH(g); He(g) b. H₂O(s); H₂O(1); H₂O(g) C. CH4(g); CF4(g); CC14(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a He Ne SO 2 NH 3 CH 3 CH 2 OH From He to Ne there is an ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Rank each set of substances in order of increasing standard molar entropy (S). Explain your reasoning. a. 1(g); F(g); Br2(g); Cl(g) b. HO(g); HO(g); HS(g) c. C(s, graphite); C(s, diamond); C(s,...
-
Rank each set of compounds in order of increasing acidity: a. b.
-
Rank each set of compounds in order of increasing boiling points. (a) Triethylamine, di-n-propylamine, n-propyl ether (b) Ethanol, dimethylamine, dimethyl ether (c) Diethylamine, diisopropylamine,...
-
What are the costs of healthcare, where does the money come from, and where is it spent?
-
Discuss the economic and trade importance of the big emerging markets.
-
The Jewel Fool had the following inventory items on hand at the end of the year. Determine the lower of cost or market per unit and the total amount that should be reported on the balance sheet for...
-
Left-tailed test, n = 6, a = 0.05 In Exercises 57-60, use a x-test to test the claim about the population variance o or standard deviation or at the given level of significance a and using the given...
-
Iowa has just passed a law making it mandatory to have every chicken inspected at least once a year for a variety of communicable diseases. Cluck Enterprises is considering entering this inspection...
-
Sunland Inc. initiated a major company expansion on January 4. 2025. During the first quarter of 2025, Sunland acquired several assets that were placed into production on July 1, 2025. The following...
-
Use data from Appendix IIB to calculate S rxn for each of the reactions. In each case, try to rationalize the sign of S rxn . Appendix IIB a. CH4(g) + H(g) CH6(g) b. C(s) + HO(g) CO(g) + H(g) c....
-
What is the molar entropy of a pure crystal at 0 K? What is the significance of the answer to this question?
-
Many tongues have been injured by licking a piece of metal on a very cold day. Why would no harm result if a clean piece of wood were licked on the same day?
-
The University of Cincinnati Center for Business Analytics is an outreach center that collaborates with industry partners on applied research and continuing education in business analytics. One of...
-
For a data set of the pulse rates for a sample of adult females, the lowest pulse rate is 38 beats per minute, the mean of the listed pulse rates is x = 78.0 beats per minute, and their standard...
-
A student earned grades of A, C, B, A, and D. Those courses had these corresponding numbers of credit hours: 5, 3, 4, 3, and 2. The grading system assigns quality points to letter grades as follows:...
-
Ch 3: Forecasting: Tracking Signals, Mad, Exponential Smoothing, Control Charts Media Consultants (10 Pts). Media Consultants uses proven techniques to measure forecast accuracy and to determine when...
-
Question 2 What is the energy (in joules) of the photon absorbed by a hydrogen atom to cause a ground-state electron to move to the n = 3 energy level? Record your answer in scientific notation to 3...
-
In accounting for a Type B lease, how are the lessee's and lessor's income statements affected?
-
The following information is available for Partin Company: Sales $598,000 Sales Returns and Allowances 20,000 Cost of Goods Sold 398,000 Selling Expense 69,000 Administrative Expense 25,000 Interest...
-
Construct a graph of the angular velocity of a car wheel as a function of time. (a) Assume the wheel starts from rest and moves with a constant (center of mass) velocity. (b) Assume the car starts...
-
Two crates of mass 5.0 kg and 9.0 kg are connected by a rope that runs over a pulley of mass 4.0 kg as shown in Figure P8.57.? (a) Make a sketch showing all the forces on both crates and the pulley.?...
-
Two crates of mass m 1 = 15 kg and m 2 = 25 kg are connected by a cable that is strung over a pulley of mass m pulley = 20 kg as shown in Figure P8.58. There is no friction between crate 1 and the...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


