Use data from Appendix IIB to calculate S rxn for each of the reactions. In each case,
Question:
Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn.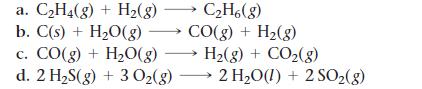
Appendix IIB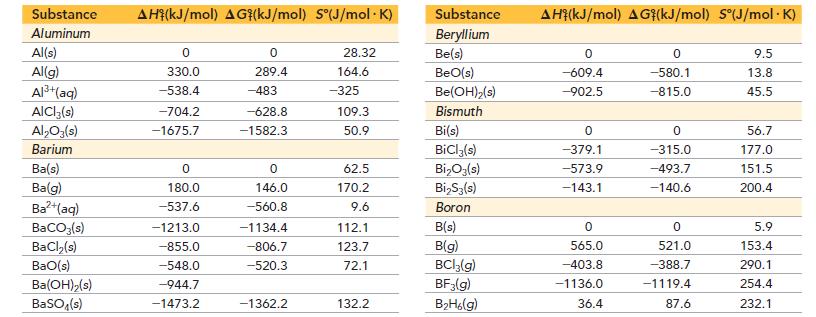
Transcribed Image Text:
a. C₂H4(g) + H₂(g) →→→ C₂H6(g) b. C(s) + H₂O(g) →→→ CO(g) + H₂(g) c. CO(g) + H₂O(g) → H₂(g) + CO₂(g) d. 2 H₂S(g) + 3 O₂(g) → 2 H₂O(l) + 2 SO₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a 1208 JK decrease in moles of g...View the full answer

Answered By

Kennedy Odhiambo
As a professional writer, I have been in the field for over 5 years having worked as a lecture in different tertiary institutions across the world. With this impeccable experience, I assure provision of a good and supporting environment for students to learn.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use data from Appendix IIB to calculate S rxn for each of the reactions. In each case, try to rationalize the sign of S rx n . Appendix IIB a. 3 NO(g) + HO(1) b. CrO3(s) + 3 CO(g) c. SO(g) + O(g) 2...
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. G f for BrCl(g) is -1.0 kJ/mol. Appendix IIB a. 2 NO(g) NO4(8) b. Br(g) + Cl(g) = 2 BrCI(g)
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. Appendix IIB a. 2 CO(g) + O(g) = 2 CO(g) b. 2 HS(g) = 2 H(g) + S(g)
-
Recession is defined as... a) increase in unemployment b) decrease in consumer spending c) two consecutive quarters of negative economic growth d) both A & B
-
One of the ramifications of emerging markets is the creation of a middle class. Discuss.
-
Emilys Greenhouse Corporation is a local greenhouse organized 10 years ago as a corporation. The bookkeeper prepared the following statement (assume that all amounts are correct, but note the...
-
Right-tailed test, n = 51, a = 0.10
-
Suppose that the size of pebbles in a river bed is normally distributed with mean 12.1mm and standard deviation 3.2 mm. A random sample of 9 pebbles are measured. Let denote the average size of the...
-
A lease has a term of 4 years and has annual payments of $3,400. The leased asset would cost $11,300 to buy and would be depreciated straight-line to a zero salvage value over 4 years. The actual...
-
Rank each set of substances in order of increasing standard molar entropy (S). Explain your reasoning. a. 1(g); F(g); Br2(g); Cl(g) b. HO(g); HO(g); HS(g) c. C(s, graphite); C(s, diamond); C(s,...
-
Rank each set of substances in order of increasing standard molar entropy (S). Explain your reasoning. a. NH3(g); Ne(g); SO(g); CH3CHOH(g); He(g) b. HO(s); HO(1); HO(g) C. CH4(g); CF4(g); CC14(g)
-
Consider the following statement: In a union shop, newly hired employees must join the union within 30 days of starting work. Forcing workers to join a union in order to keep their job is...
-
2. See US Debt Clock and answer the following: (Hint: Take a screenshot of the Debt Clock) (2) A. What is the current US deficit and the total federal debt? (1) B What is the net interest...
-
Q. Is GDP per capita a good measure of a society's welfare? Why or why not? (150 Words)
-
On May 3, the Happy Company wrote off the $4,300 uncollectible account of its customer, A. Johnson. The entry or entries Happy makes to record the write off of the account on May 3 is: Allowance for...
-
Who is responsible for the financial statements and maintaining effective internal control over financial reporting? Where did you find this in the annual report? What accounting rules are required...
-
8. Chad owned an office building that was destroyed in a tornado. The adjusted basis of the building at the time was $890,000. After the deductible, Chad received an insurance check for $850,000. He...
-
In a Type A lease, "front loading" of lease expense and lease revenue occurs. What does this mean, and how is it avoided in a Type B lease?
-
1. What are some current issues facing Saudi Arabia? What is the climate for doing business in Saudi Arabia today? 2. Is it legal for Auger's firm to make a payment of $100,000 to help ensure this...
-
A ceiling fan of radius 45 cm runs at 90 rev/min. How far does the tip of the fan blade travel in 1 hour?
-
Construct a plot of the angular displacement of a CD as a function of time. Use information from this chapter to attach quantitative labels (numbers and units) to your graph. Take t = 0 to be the...
-
A potters wheel of radius 0.20 m is turned on at t = 0 and accelerates uniformly. After 60 s, the wheel has an angular velocity of 2.0 rad/s. Find the angular acceleration and the total angular...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


