Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. G
Question:
Use data from Appendix IIB to calculate the equilibrium constants at 25 °C for each reaction. ΔG°f for BrCl(g) is -1.0 kJ/mol.![]()
Appendix IIB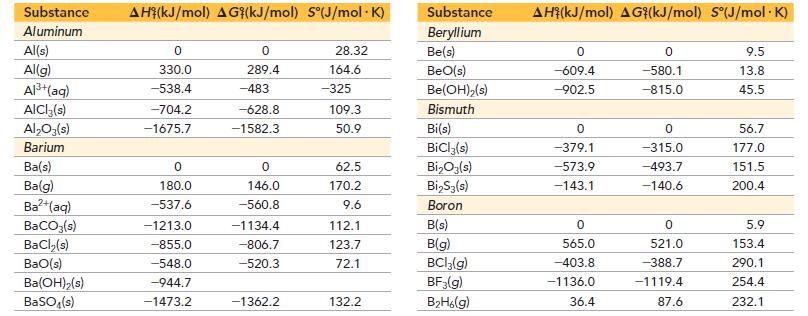
Transcribed Image Text:
a. 2 NO₂(g) N₂O4(8) b. Br₂(g) + Cl₂(g) = 2 BrCI(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To calculate the equilibrium constant K at 25 C for a given reaction from standard Gibbs free energy of formation Gf values you can use the following ...View the full answer

Answered By

Mahesh G
I have more than 7 years of experience in teaching physics, mathematics and python programming to more than 600 students including both online and offline tutoring.
I follow the following 7 step fundamental approach towards tutoring.
1. Curiosity, scope, enlightenment of the topic in hand.
2. Problem Definitions and elaboration.
3. Requisite mathematics, analytical abilities and quantitative
aptitude.
4. Preparing Algorithms for problem statement.
5. Concepts with analogies and building algorithm.
6. Introspection and improvising.
7. Daily class wise Cheat sheets(its not cheating) for consolidation.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. Appendix IIB a. 2 CO(g) + O(g) = 2 CO(g) b. 2 HS(g) = 2 H(g) + S(g)
-
Estimate the value of the equilibrium constant at 655 K for each reaction in Problem 74. (H f for BrCl is 14.6 kJ/mol.) Problem 74 Use data from Appendix IIB to calculate the equilibrium constants at...
-
Use data from Appendix C to calculate the equilibrium constant, K, at 298 K for each of the following reactions: H2(g) + 12(g) 2 HI(g) C2H5OH (g)- C2H4(g) + H2O(g)
-
1. A projectile is launched in a vertical plane, at an angle 0 with initial velocity vo. It must be caught in a frictionless circular tube of radius R in such a way that the trajectory of the...
-
Bell Printing Company specializes in wedding in wedding invitations. Bell needs information to budget next years activities. Write yes or no to indicate whether each of the following costs is likely...
-
Let X1, X2, . . ., X10 be a random sample from a standard normal distribution. Find the numbers a and b such that 1
-
How might this affect the risk-taking behaviour of directors?
-
Bit and Byte sells computer services to its clients. The firm is contemplating the acquisition of a computer but is undecided whether it should be leased or purchased. Information regarding the...
-
Mr and Mrs Netyu run a private company, Netyu Toys Ltd . Toys are imported from China. Mr Netyu has asked you to prepare set of financial statements for the year ended 3 1 December 2 0 2 2 as soon as...
-
Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following conditions: a. Standard conditions b. At equilibrium c. P CH3OH = 1.0 atm; P CO = P H2 = 0.010 atm CO(g) +...
-
Which process results in the increase in entropy of the universe? a) The cooling of a hot cup of coffee in room temperature air b) The evaporation of water from a desk at room temperature c) The...
-
In Exercises 2142, evaluate each expression without using a calculator. log4 1
-
Based on the reading,How to make sure your next product or service launch drives growth (click the underlined link),what stands out to you as the most important factor in a differentiated launch...
-
OM in the News has previously looked at the Waffle House Index, used to measure the damage from hurricanes. The index made the news again for Hurricane Ian. According to the Boston Globe, 40 Waffle...
-
Mixture of persuasive and negative formal I am Elizabeth grinderFirst part email Next part setting up the meeting Final part memo The reader is Robert * do not come off accusatory*** Project TWO:...
-
Anyone who has sampled today's social media offerings has probably experienced this situation: You find a few fascinating blogs, a few interesting people to follow on Twitter, a couple of podcast...
-
a. Begin with a converging lens of focal length f. Place an illuminated object a distance p, in front of the lens. For all positive values of p;: 1. calculate and sketch a graph of the location of...
-
Stockholders' equity totaled $164,000 at the beginning of the year. During the year, net income was $24,000, dividends of $6,000 were declared and paid, and $20,000 of common stock was issued at par...
-
What does non-recourse financing mean?
-
If the frequency of a wave is doubled, how does the wavelength change?
-
A solid glass sphere with an initial diameter d = 25.00 cm is subject to a pressure of 1000 P atm , where atmospheric pressure P atm = 1.01 10 5 Pa. What is the new diameter of the sphere? The...
-
A shear force of 3.0 ? 10 5 N is applied to an aluminum bar of length L = 20 cm, width w = 5.0 cm, and height h = 2.0 cm as shown in Figure 11.23 . What is the shear deformation??x? Figure 11.23 ? A...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


