Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. Appendix
Question:
Use data from Appendix IIB to calculate the equilibrium constants at 25 °C for each reaction.![]()
Appendix IIB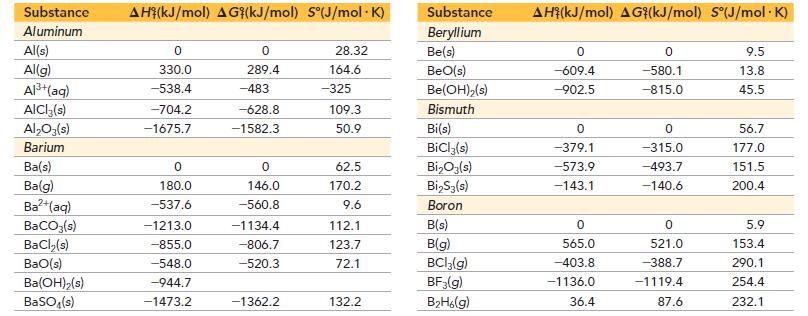
Transcribed Image Text:
a. 2 CO(g) + O₂(g) = 2 CO₂(g) b. 2 H₂S(g) = 2 H₂(g) + S₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a 148...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. G f for BrCl(g) is -1.0 kJ/mol. Appendix IIB a. 2 NO(g) NO4(8) b. Br(g) + Cl(g) = 2 BrCI(g)
-
Estimate the value of the equilibrium constant at 655 K for each reaction in Problem 74. (H f for BrCl is 14.6 kJ/mol.) Problem 74 Use data from Appendix IIB to calculate the equilibrium constants at...
-
Use data from Appendix C to calculate the equilibrium constant, K, at 298 K for each of the following reactions: H2(g) + 12(g) 2 HI(g) C2H5OH (g)- C2H4(g) + H2O(g)
-
8. A 12-tur square wire loop of 1-m length carrying a current of 1-A in the counter clockwise direction as shown in the figure. A magnetic field of magnitude 3000 G is directed into the board. Find,...
-
For each of the following transactions, state which account (s) would be debited and credited in a job order costing system for a desert landscaping business: 1. Charged customer for landscape design...
-
Suppose a coin is tossed 100 times in order to estimate p = p (Head). It is observed that head appeared 60 times. Find a 95% confidence interval for p.
-
Discuss the advantages and disadvantages of each of the main forms of purchase consideration used in a takeover.
-
Shadow Corp. has no debt but can borrow at 8 percent. The firms WACC is currently 11 percent, and the tax rate is 35 percent. a. What is Shadows cost of equity? b. If the firm converts to 25 percent...
-
Question 2 The management of a company wishes to window-dress its cash flow from operations. Which of the following will improve cash flow from operations? I Factoring accounts receivable II Paying...
-
Which process results in the increase in entropy of the universe? a) The cooling of a hot cup of coffee in room temperature air b) The evaporation of water from a desk at room temperature c) The...
-
Without doing any calculations, determine the signs of S sys and S sur r for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high...
-
The activity of a radioactive source decreases by 5.5% in 31.0 hours. What is the half-life of this source?
-
15.5 please help will give like if answers r correct Exercise 15-8 (Static) Sales-type lease with selling profit; lessor; calculate lease payments [LO15-3] Manufacturers Southern leased high-tech...
-
When my son was young, he had 8 different plastic dinosaurs to arrange. How many ways could he arrange his 8 dinos? He had favorite dinos, so placing them in proper order was very important. How many...
-
Process P1 init (mutEx); num = 0; loop1 = 0; while (loop1 < 3) wait (mutEx); num num + 1; signal (mutEX); loop1 loop1 + 1; Process P2 loop2 = 0; while (loop2 < 2) wait (mutEx); num num + 10;...
-
PROBLEM 3-5B Following is the chart of accounts of Smith Financial Services: Assets 111 Cash 113 Accounts Receivable 115 Supplies 117 Prepaid Insurance 124 Office Furniture Liabilities 221 Accounts...
-
4. Identify a service you could refer Casey to and write a referral for her (up to 300 words).
-
Gary's TV had the following accounts and amounts in its financial statements on December 31, 2016. Assume that all balance sheet items reflect account balances at December 31. 2016, and that all...
-
Complete problem P10-21 using ASPE. Data from P10-21 Original cost ................................................................. $7,000,000 Accumulated depreciation...
-
A solid sphere and a solid cylinder, both of mass m and radius R, are rolling without slipping with speed v. Find (a) The ratio of the angular momentum of the sphere to that of the cylinder and (b)...
-
An object is rolling without slipping, with a transnational kinetic energy of KE trans and a rotational kinetic energy of KE rot . If KE trans / KE rot = 3/2, what might this object be, (a) a hoop,...
-
A figure skater (Fig. 9.11) has an initial angular velocity of 3.8 rad/s. She then pulls her arms in, and her angular velocity increases to 9.5 rad/s. What is the ratio of her final kinetic energy to...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


