Refer to the K sp values in Table 18.2 to calculate the molar solubility of each compound
Question:
Refer to the Ksp values in Table 18.2 to calculate the molar solubility of each compound in pure water.
a. AgBr
b. Mg(OH)2
c. CaF2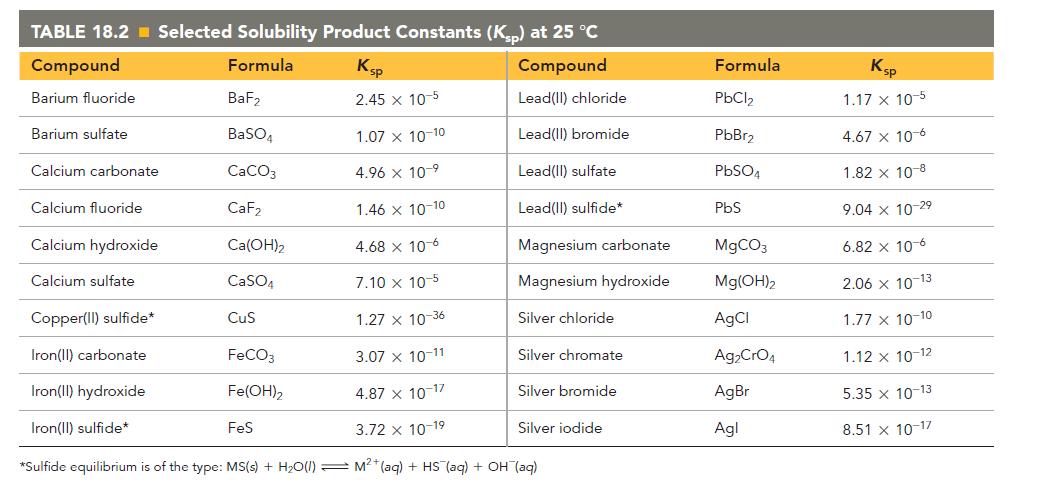
Transcribed Image Text:
TABLE 18.2 Selected Solubility Product Constants (Ksp) at 25 °C Compound Formula Ksp Compound Barium fluoride 2.45 x 10-5 Lead(II) chloride Lead(II) bromide Lead(II) sulfate Lead(II) sulfide* Barium sulfate Calcium carbonate Calcium fluoride Calcium hydroxide Calcium sulfate BaF₂ BaSO4 CaCO3 CaF₂ 1.46 x 10-10 4.68 x 10-6 7.10 x 10-5 Copper(II) sulfide* 1.27 x 10-36 Iron(II) carbonate 3.07 x 10-11 Iron(II) hydroxide 4.87 x 10-17 Iron(II) sulfide* 3.72 x 10-19 *Sulfide equilibrium is of the type: MS(s) + H₂O(1) M²+ (aq) + HS (aq) + OH(aq) Ca(OH)₂ CaSO4 CuS FeCO3 Fe(OH)2 1.07 x 10-10 4.96 x 10-⁹ FeS Magnesium carbonate Magnesium hydroxide Silver chloride Silver chromate Silver bromide Silver iodide Formula PbCl₂ PbBr₂ PbSO4 PbS MgCO3 Mg(OH)2 AgCl Ag₂CrO4 AgBr Agl Ksp 1.17 x 10-5 4.67 x 10-6 1.82 x 10-8 9.04 x 10-2⁹ 6.82 x 10-6 2.06 x 10-13 1.77 x 10-10 1.12 x 10-12 5.35 x 10-13 8.51 x 10-17
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
a 731 10 ...View the full answer

Answered By

Vincent Omondi
I am an extremely self-motivated person who firmly believes in his abilities. With high sensitivity to task and operating parameters, deadlines and keen on instructions, I deliver the best quality work for my clients. I handle tasks ranging from assignments to projects.
4.90+
109+ Reviews
314+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Refer to the K sp values in Table 18.2 to calculate the molar solubility of each compound in pure water. a. MX (Ksp = 1.27 * 10 -36 ) b. Ag 2 CrO 4 c. Ca(OH) 2 TABLE 18.2 Selected Solubility Product...
-
Compound Ksp Iron(II) sulfide 3.7210-19 Magnesium hydroxide 2.0610-13 Lead(II) bromide 4.6710-6 A) Use the ksp values in the table to calculate the molar solubility of FeS in pure water. S= ____M...
-
Tooth enamel is composed of hydroxyapatite, whose simplest formula is Ca 5 (PO 4 ) 3 OH, and whose corresponding Ksp = 6.8 10 -27 .As discussed in the Chemistry and Life box on page 730, fluoride in...
-
An LNC can detect alterations in the record by Select one: a. Examining notes written in the margin b. Looking through late entries for detailed explanations c. Finding a doctor's explanation that is...
-
Murphy Company manufactures and sells three products. Relevant per unit data concerning each product are given below. Instructions (a) Compute the contribution margin per unit of the limited resource...
-
In Exercise 5.21 we saw, among other things, the weight gain of each of 29 anorexic girls who received cognitive behavioral therapy. What null hypothesis would we likely be testing in this situation?
-
E 22-9 College journal entries Burges Colleges total tuition and fees is at $1,250,000, where a fellowship program reduces the fees by 5 percent, and a bad debt of 2.5 percent is expected. The...
-
Saddle Inc. has two types of handbags: standard and custom. The controller has decided to use a plant-wide overhead rate based on direct labor costs. The president has heard of activity-based costing...
-
Consider the following abbreviated financial statements for Parrothead Enterprises: a . What is owners' equity for 2 0 2 0 and 2 0 2 1 ? ( Do not round intermediate calculations and round your answer...
-
Write balanced equations and expressions for K sp for the dissolution of each ionic compound. a. CaCO 3 b. PbCl 2 c. AgI
-
Methyl red has a pK a of 5.0 and is red in its acid form and yellow in its basic form. If several drops of this indicator are placed in a 25.0-mL sample of 0.100 M HCl, what color will the solution...
-
The double pulley shown is attached to a 0.5-in.-radius shaft which fits loosely in a fixed bearing. Knowing that the coefficient of static friction between the shaft and the poorly lubricated...
-
Determine the magnitude of the magnetic flux through the south-facing window of a house in British Columbia, where Earth's B field has a magnitude of 5.8 x 10-5T and the direction of B field is 72...
-
A wedge with an inclination of angle rests next to a wall. A block of mass m is sliding down the plane, as shown. There is no friction between the wedge and the block or between the wedge and the...
-
Conner Leonard worked for Purges Manufacturing for 32 years. Along with four other men, he helped to start the company that designed and built products sold around the world. Purges Manufacturing...
-
Reconsider the collision between two objects diagrammed below where two objects move on a frictionless surface. Before collision After collision Experiment 1 A, 1 B A B Draw complete and properly...
-
3. Now the bomb arrives. Please catch fx,y(x, y) = = cx cx - dy, where 0 < x < 1, 0 y x. 13 a) Please find coefficients c, d such that cd= 8 b) Please find fx(x) and fy (y). Are X and Y independent?...
-
Information for Gibbs Corporation is given in E8-7. In E8-7 Gibbs Corporation produces industrial robots for high-precision manufacturing. The following information is given for Gibbs Corporation....
-
Without solving, determine the character of the solutions of each equation in the complex number system. 3x 2 3x + 4 = 0
-
You have a solution that contains Ca 2+ ions and another that contains Na + ions. How would adding a solution that contains CO 2- 3 ionss enable you to tell which is which?
-
What is the difference between a molecular ion and a polar molecule?
-
Ordinary tap water tastes different after it has been boiled. Can you think of the reason why?
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...

Study smarter with the SolutionInn App


