Refer to the K sp values in Table 18.2 to calculate the molar solubility of each compound
Question:
Refer to the Ksp values in Table 18.2 to calculate the molar solubility of each compound in pure water.
a. MX (Ksp = 1.27 * 10-36)
b. Ag2CrO4
c. Ca(OH)2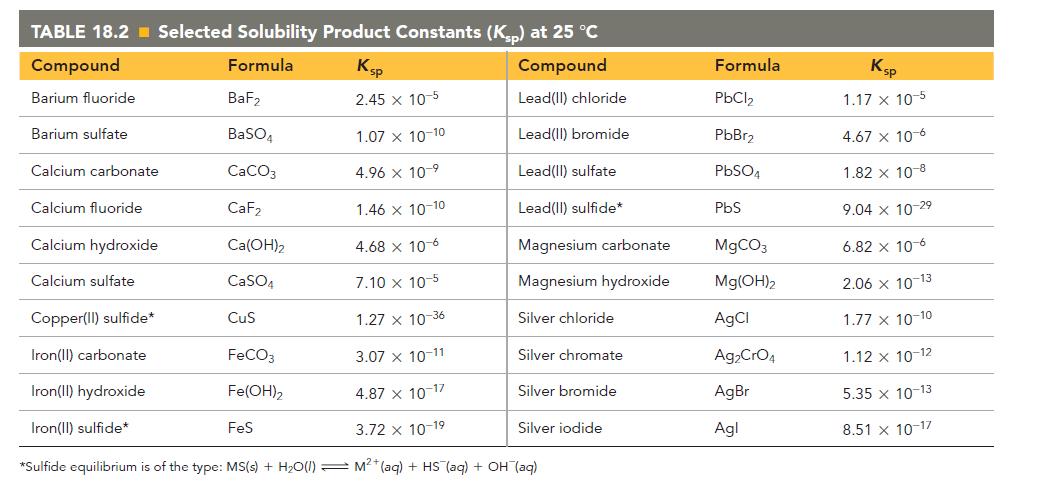
Transcribed Image Text:
TABLE 18.2 Selected Solubility Product Constants (Ksp) at 25 °C Compound Formula Ksp Compound Barium fluoride 2.45 x 10-5 Lead(II) chloride Lead(II) bromide Lead(II) sulfate Lead(II) sulfide* Barium sulfate Calcium carbonate Calcium fluoride Calcium hydroxide Calcium sulfate BaF₂ BaSO4 CaCO3 CaF₂ Ca(OH)₂ CaSO4 1.46 x 10-10 4.68 x 10-6 7.10 x 10-5 Copper(II) sulfide* 1.27 x 10-36 Iron(II) carbonate 3.07 x 10-11 Iron(II) hydroxide 4.87 x 10-17 Iron(II) sulfide* 3.72 x 10-19 *Sulfide equilibrium is of the type: MS(s) + H₂O(1) M²+ (aq) + HS (aq) + OH(aq) CuS FeCO3 Fe(OH)2 1.07 x 10-10 4.96 x 10-⁹ FeS Magnesium carbonate Magnesium hydroxide Silver chloride Silver chromate Silver bromide Silver iodide Formula PbCl₂ PbBr₂ PbSO4 PbS MgCO3 Mg(OH)2 AgCl Ag₂CrO4 AgBr Agl Ksp 1.17 x 10-5 4.67 x 10-6 1.82 x 10-8 9.04 x 10-2⁹ 6.82 x 10-6 2.06 x 10-13 1.77 x 10-10 1.12 x 10-12 5.35 x 10-13 8.51 x 10-17
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
To calculate the molar solubility of each compound in pure water we need to use the Ksp expression K...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Refer to the K sp values in Table 18.2 to calculate the molar solubility of each compound in pure water. a. AgBr b. Mg(OH) 2 c. CaF 2 TABLE 18.2 Selected Solubility Product Constants (Ksp) at 25 C...
-
Compound Ksp Iron(II) sulfide 3.7210-19 Magnesium hydroxide 2.0610-13 Lead(II) bromide 4.6710-6 A) Use the ksp values in the table to calculate the molar solubility of FeS in pure water. S= ____M...
-
Refer to Table 10.1 in the text and look at the period from 1973 through 1978. a. Calculate the arithmetic average returns for common stocks and T-bills over this period. b. Calculate the standard...
-
Consider the following table of countries and their associated maximum production possibilities for wheat and wool below. Note that these numbers represent tonnes of output per day. Country Wheat...
-
Alameda Service Center just purchased an automobile hoist for $15,000. The hoist has a 5-year life and an estimated salvage value of $1,080. Installation costs were $2,900, and freight charges were...
-
Calculate the adjusted R2 for the 15 cases in Exercise 11.6. Twice in this chapter I said that we were going to ignore the adjusted R2, even though it is a perfectly legitimate statistic. Can you...
-
E 22-8 Expense classification at a not-for-profit college The expenses at a college are as follows: 1. Computer laboratory maintenance 2. Faculty salary 3. Student consultation 4. Research grants for...
-
Central Ski and Cycle purchased 50 pairs of ski boots for $360 per pair less 33 % and 10%. The regular rate of markup on selling price of the boots is 40%. The stores overhead is 22% of the selling...
-
Analytics is now at the forefront of the insurance industry. Please describe the applicability of analytics to managing, retaining and transferring risk. Describe briefly the tools that are being...
-
Write balanced equations and expressions for K sp for the dissolution of each ionic compound. a. CaCO 3 b. PbCl 2 c. AgI
-
Methyl red has a pK a of 5.0 and is red in its acid form and yellow in its basic form. If several drops of this indicator are placed in a 25.0-mL sample of 0.100 M HCl, what color will the solution...
-
Jenny ran 3 1/3 miles on Saturday and 2 4/5 miles on Sunday. The total distance, in miles, Jenny ran during those 2 days is within which of the following ranges? A. At least 6 1/2 and less than 6 2/3...
-
7. A psychiatrist is testing a new ADHD Medication, which seems to have the potentially harmful side effect of increasing the heart rate. For a sample of 50 clinical study participants whose pulse...
-
Determine the type of engagement that your colleague completed for the client. Justify the selected engagement type for the client. Assess the purpose of each financial statement for the client's...
-
Mills Corporation acquired as a long-term investment $235 million of 8% bonds, dated July 1, on July 1, 2024. Company management has classified the bonds as an available-for-sale investment. The...
-
A force of 28 pounds acts on the pipe wrench shown in the figure below. 18 in. 30 (a) Find the magnitude of the moment about O by evaluating ||OA x F||. (0 0 180) Use a graphing utility to graph the...
-
Module 1 1. There has been a rise in cases of measles in RI. The RI Health Department is wondering if the rate of MMR vaccinations has declined since the start of the COVID-19 pandemic. The...
-
Leno Company makes swimsuits and sells these suits directly to retailers. Although Leno has a variety of suits, it does not make the All-Body suit used by highly skilled swimmers. The market research...
-
Interview managers at three companies in your area about their use of ERP. How have their experiences been similar? What accounts for the similarities and differences?
-
What are the two chief ions found in seawater?
-
Seawater freezes at a lower temperature than pure water because of the salts dissolved in it. How does the boiling point of seawater compare with that of pure water?
-
What is the easiest way to distinguish between a solution that contains Cu 2+ ions from one that contains Ca 2+ ions?
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
Quantum Language And The Migration Of Scientific Concepts 1st Edition - ISBN: 0262037556 - Free Book

Study smarter with the SolutionInn App


