Refer to the periodic table to write the electron configuration for selenium (Se). Orbital Blocks of the
Question:
Refer to the periodic table to write the electron configuration for selenium (Se).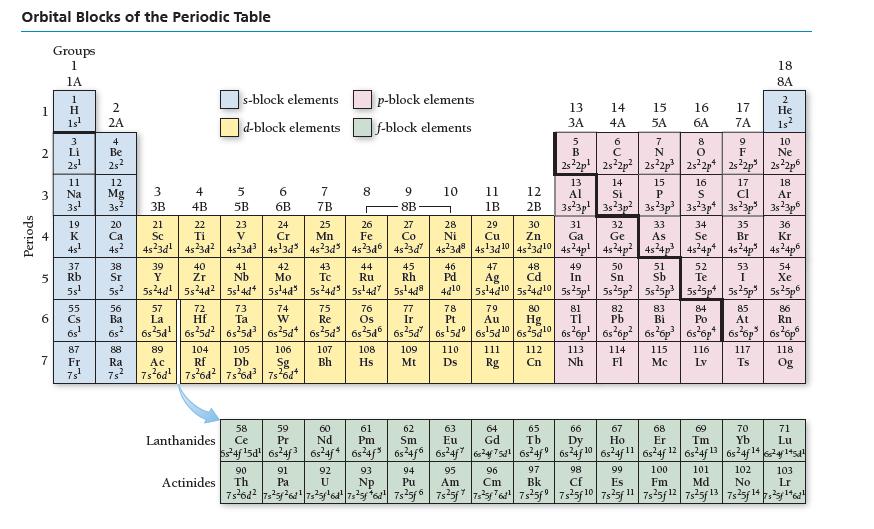
Transcribed Image Text:
Orbital Blocks of the Periodic Table Periods 1 2 3 4 6 Groups 1 1A 7 4x523=~=- 2 1s¹ 2A 25² Na 37 5 Rb 5s¹ 12 Mg 3s¹ 35² 20 Ca 4s¹ 45² 55 Cs 6s¹ 4 Be Fr 25²2 ܐܨܐ 38 Sr 55² 56 Ba 68 2 88 Ra 3 3B 39 Y 5s²4d¹ 4 4B 57 La 40 Zr 5s²4d² s-block elements d-block elements 5 5B 72 Hf 23 V 6 6B 43 41 Nb Tc 5s¹4d4 5s¹4d³ 5s²4d" 105 89 106 104 Ac Rf 7s²6d¹ 7s²6d² 7s²6d³ 7s²6d+ Db Sg 2 42 Mo 58 Lanthanides Ce 7 7B 90 Actinides Th 25 Mn 00 91 Pa 8 107 Bh p-block elements f-block elements 44 Ru 5s¹4d7 9 8B 108 Hs 60 61 62 59 Pr Pm Nd Sm 6s24f¹5d 6s24f3 6s24j4 6s²4f³ 6s²4f6 45 Rh 5s¹4d8 10 21 22 24 26 27 31 32 33 34 35 36 Sc Ti Cr Fe Co Ga Ge As Se Br Kr 4s²3d¹ 45²3d² 4s²3d³4s¹3d³4s²3d³ 45²3d6 45²3d² 4s²3d8|4s¹3d¹0 4s²3d¹0 4s²4p² 4s²4p² 4s²4p³ 45²4p² 4s²4p³ 4s²4p6 93 NP 28 Ni 11 46 Pd 1B 109 110 Mt Ds 29 Cu 47 Ag 289338278 79 Au 12 111 Rg 2B 30 96 Cm Zn 48 Cd 73 76 77 78 81 84 85 86 74 W 75 Re Ta Os Ir Pt TI Po At Rn 6s²5d¹|6s²5d² 6s²5d³ 6s²5d 6s²5d³ 6s²5d 6s²5d 6s¹5d 6s¹5d1065²5d¹0 6s²6p¹ 6s²6p²6s²6p³ 6s²6p 6s²6p³ 6s¹6p6 80 13 14 3A 4A Hg SA. 63 64 Eu Gd 6s²4f7 624f75d¹6s²4f9 95 Am 65 Tb 5 49 50 52 54 In Sn Te Xe 4d10 5s4d10 5s24d10 5s25p² 5s25p² 5s²5p³ 5s²5p 5s25p³ 5s²5p6 B OU. 8 9 O F 2s²2p¹ 2s²2p² 2s²2p3 2s²2p4 2s22p 2s²2p6 15 5A 112 113 114 Nh Fl Cn 82 Pb 97 98 Bk Cf 7 N ទី 13 17 18 14 Si 15 P Al 16 S cl Ar 3s 3p 3s23p² 3s23p³ 3s²3p4 3s²3p 3s²3p6 16 6A 67 Ho 17 7A 51 Sb 83 Bi 115 Mc 18 8A 2 He 1s² 53 I 10 Ne 116. 117 LV Ts 102 No 66 68 70 69 Dy Er Tm Yb Lu 6s²4f 10 6s²4f1165-4f 12 6s24f 13 6s24f 14624145d1 101 92 U 94 Pu 99 Es 100 Fm Md 7s²6d² 7s²5f²6d¹|7s²5f¹6d²|7s²5f6d¹ 7s²5f6 7s²5f7s²5f76d²¹ 7s²5f9|7s²5f 10 7s25f 1¹1 7s25f 12 7s²5f 13 7s25f14725¹46¹ 118 Og 71 EG 103 Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
The atomic number of Se is 34 The noble gas that precedes Se in the ...View the full answer

Answered By

Sandip Agarwal
I have an experience of over 4 years in tutoring. I have solved more than 2100 assignments and I am comfortable with all levels of writing and referencing.
4.70+
19+ Reviews
29+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What are the costs associated with the execution of a database request in a distributed database. Describe three of them Describe three examples where a graph database will be more suitable than a...
-
Use the periodic table to write an electron configuration for each element. Represent core electrons with the symbol of the previous noble gas in brackets. a. P b. Ge c. Zr d. I
-
A case of 12 boxes of macaroni and cheese can be purchased for $18.00. How much is each box of macaroni and cheese?
-
Under which of the following circumstances would an auditor be most likely to intensify an challenging examination of a $500 imprest petty cash fund a. Reimbursement occurs twice each week. b. The...
-
Melton Corporation has a current ratio of 1.1. Sam has always been told that a corporations current ratio should exceed 2.0. Melton argues that its ratio is low because it has a minimal amount of...
-
Seeprint Limited is negotiating an initial one year contract with an important customer for the supply of a specialized printed colour catalogue at a fixed contract price of 16 per catalogue....
-
1. Assign roles in your group. These roles may stay the same or may rotate among team members to provide an opportunity for each team member to practice each role. Select a leader: The leader is...
-
For the coming year, Bernardino Company anticipates a unit selling price of $85, a unit variable cost of $15, and fixed costs of $420,000. Instruction 1. Compute the anticipated break-even sales...
-
Mrs Tredoux is a monthly employee. She is 40 years old and has 2 beneficiaries on her medical aid excluding herslef. Mrs Tredoux receives a monthly travel allowance of R2050. She travelled 9074km in...
-
A network administrator has received reports of intermittent connectivity issues. To diagnose the problem, the network administrator has decided to use tcpdump. Which of the following are the primary...
-
Explain the contributions of Johann Dbereiner and John Newlands to the organization of elements according to their properties.
-
What are the four quantum numbers for each of the two electrons in a 4s orbital? (a) n = 4, l = 0, m l = 0, m s = +; n = 4, l = 0, m l = 0, m s = + (b) n = 4, l = 0, m l = 0, m s = +; n = 4, l = 0, m...
-
H.C. Blackwell Co. held a franchise from Kenworth Truck Co. to sell its trucks. After 12 years, the franchise was nearing expiration. Kenworth notified Blackwell that the franchise would not be...
-
1. first order of business is to create your city. What will you name your city? Name of City: 2. What are the main economic goals? Choose 2-3 goals . Why did choose these particular goals? What...
-
The organization I am presenting today is Tyler Coca-Cola Bottling, located in Tyler, Texas. Tyler Coca-Cola operates under the guidance of Coca-Cola Southwest Beverages. Tyler, Texas is a small...
-
Explain the interplay between power dynamics and communication patterns in hierarchical organizations. How do these factors influence decision-making and innovation?
-
Adidas-Consumer Goods STEP ONE: MISSION: Mission statement core message that guides and influences your marketing strategy. Why is this company in business and what is the purpose of their...
-
You have been operating and growing your golf club for the last six (6) years. You are happy with the fact that all revenue streams (and as a result your share value) have continued to increase as...
-
Roberts Corporation developed the following standard unit costs at 100% of its normal production capacity, which is 50,000 units per year: Direct materials...
-
The Alert Company is a closely held investment-services group that has been very successful over the past five years, consistently providing most members of the top management group with 50% bonuses....
-
In discussing the Boltzmann distribution in Chapter 13, we used the symbols gi and gj to indicate the degeneracies of the energy levels i and j. By degeneracy, we mean the number of distinct quantum...
-
Show by examining the position of the nodes that Re[A + e i(kx t) ] and Re[A e i(kx t) ] represent plane waves moving in the positive and negative x directions, respectively. The notation Re [ ]...
-
Two wave functions are distinguishable if they lead to a different probability density. Which of the following wave functions are distinguishable from sin kx? a. (e ikx e Ikx )/2 b. e i sin kx, a...
-
Lina Center for Performing Arts is a not-for-profit organization. The center began operations on January 1, 2021 with $135,000 of temporarily restricted assets. The amounts are restricted for the...
-
Singer Company has a line of credit with United Bank. Singer can borrow up to $307,000 at any time over the course of the Year 1 calendar year. The following table shows the prime rate expressed as...
-
please need urgent help with these 2 journal entries On February 3 , a company provides services on account for $26,500, terms 2/10,n/30. On February 9 , the company recelves payment from the...

Study smarter with the SolutionInn App


