Sulfuric acid (H 2 SO 4 ) is a component of acid rain that forms when SO
Question:
Sulfuric acid (H2SO4) is a component of acid rain that forms when SO2, a pollutant, reacts with oxygen and water according to the simplified reaction:![]()
The generation of the electricity used by a medium-sized home produces about 25 kg of SO2 per year. Assuming that there is more than enough O2 and H2O, what mass of H2SO4, in kg, can form from this much SO2?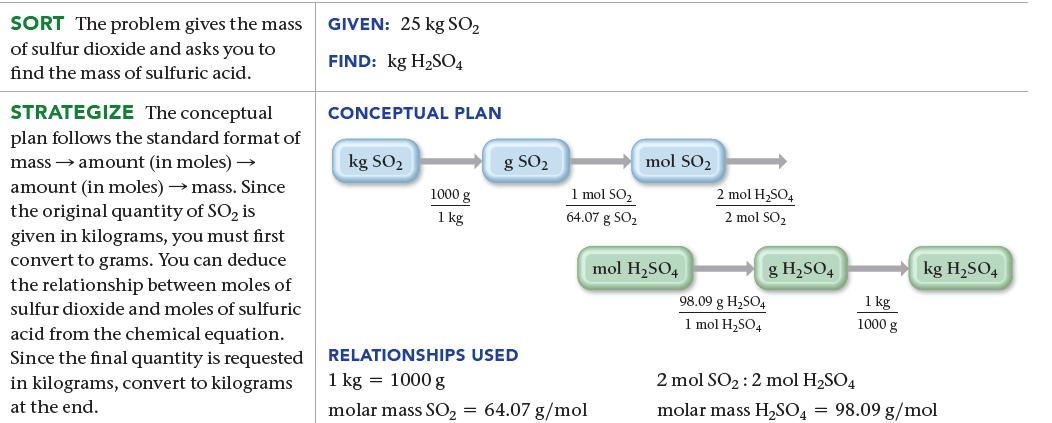
Transcribed Image Text:
2 SO₂(g) + O₂(g) + 2 H₂O(1) 2 H₂SO4(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
25 kg S X 1000 g 1 kg X 1 molSO 6407 g SO X 2 mol HSO4 2 molSO X 9809 ...View the full answer

Answered By

Vincent Omondi
I am an extremely self-motivated person who firmly believes in his abilities. With high sensitivity to task and operating parameters, deadlines and keen on instructions, I deliver the best quality work for my clients. I handle tasks ranging from assignments to projects.
4.90+
109+ Reviews
314+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Sulfuric acid (H2SO4) is a component of acid rain that forms when SO2, a pollutant, reacts with oxygen and water according to the simplified reaction: 2 SO2(g) + O2(g) + 2 H2O(l) -------> 2 H2SO4(aq)...
-
Nitric acid is a component of acid rain that forms when gaseous nitrogen dioxide pollutant reacts with gaseous oxygen and liquid water to form aqueous nitric acid. Write the balanced chemical...
-
Sulfuric acid is a component of acid rain formed when gaseous sulfur dioxide pollutant reacts with gaseous oxygen and liquid water to form aqueous sulfuric acid. Write the balanced chemical equation...
-
Researchers investigated the relationship between the number of involuntary admissions (detentions) for mental disorders a year under the Mental Health Act 1983 and the number of NHS psychiatric beds...
-
Data for Rivera Company are presented in P12-7B. Further analysis reveals the following. 1. Accounts payable pertains to merchandise creditors. 2. All operating expenses except for depreciation are...
-
An inventor claims to have developed a refrigeration system that removes heat from the closed region at -12C and transfers it to the surrounding air at 25C while maintaining a COP of 6.5. Is this...
-
Why might a company, such as Caterpillar, find advantages to evaluating bundles of investment simultaneously rather than singly, as described in Research Insight 14.1?
-
The Metro Police Department has partitioned the city into four quadrants. The department has 20 police patrol cars available for each shift per day to assign to the quadrants. The department wants to...
-
Carmen Camry operates a consulting firm called Help Today, which began operations on August 1. On August 31, the company's records show the following selected accounts and amounts for the month of...
-
Russian is an Indo-European language of the Slavic family, spoken in Russia. Determine from the following Russian data whether the low front [a] and the low back [a] complement each other as...
-
Identify the reactants and products in this chemical equation. 4 NH 3 (g) + 5 O2( g) 4 NO(g) + 6 H 2 O(g)
-
Manganese(IV) oxide reacts with aluminum to form elemental manganese and aluminum oxide: What mass of Al is required to completely react with 25.0 g MnO 2 ? a) 7.76 g Al b) 5.82 g Al c) 33.3 g Al d)...
-
Summarize ways to measure the effectiveness of human resource management.
-
A boat leaves port and follows a course of N77E at 9 knots for 3 hr and 20 min. Then, the boat changes to a new course of S26E at 12 knots for 5 hr. Part 1 of 3 (a) How far is the boat from port?...
-
The aggregate supply curve of an economy is depicted by AS, shown in the graph on the right. Suppose that labour unions grant concessions, enabling firms to pay lower wages to their workers. Use the...
-
what is Medibank pestle analysis in term of these 2 statements? Current problem at hand deviates towards the fact that customers do not have high awareness of the health and wellbeing programs that...
-
Your company has a Microsoft 365 E5 subscription. You need to review the Advanced Analysis tab on emails detected by Microsoft Defender for Office 365. What type of threat policy should you...
-
(a) The Bright company is evaluating a project which will cost Rs 1,00,000 and will have no salvage value at the end of its 5-year life. The project will save costs of Rs. 40,000 a year. The company...
-
Defco Division of Gunnco Corporation requests of Omar Division a supply of Electrical Fitting 1726, which is not available from any other source. Omar Division, which is operating at capacity, sells...
-
Havel says the grocer doesnt believe what is on the sign and indeed, he says the grocers customers will barely notice it. But Havel maintains that the sign serves a specific function. How would you...
-
Calculate the difference in pressure across the liquidair interface for a (a) Mercury and (b) Methanol droplet of radius 125 nm.
-
Calculate the vapor pressure of CH 3 OH(l) at 298.15 K if He is added to the gas phase at a partial pressure of 200. Bar using the data tables. By what factor does the vapor pressure change?
-
Use the vapor pressures of SO 2 (l) given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. T (K) 190. P (Pa) T (K) 230. P...
-
Hardwood Supplies Inc. purchased a 12-month insurance policy on March 1, 2020 for $3,000. At March 31, 2020, the adjusting journal entry to record expiration of this asset will include a debit to...
-
You are required to complete a detailed design of an office building. The geometry of the building is shown in Figures 3 and 4. Figure 3 shows an elevation view while Figure 4 shows a typical...
-
Gomer is admitted to Mouton Partnership on July 1, 2020. Gomer contributes ABC common stock purchased in 1992 for $20,000, with a fair market value of $100,000 on July 1, 2020 for a 25% interest in...

Study smarter with the SolutionInn App


