The explosive nitroglycerin (C 3 H 5 N 3 O 9 ) decomposes rapidly upon ignition or
Question:
The explosive nitroglycerin (C3H5N3O9) decomposes rapidly upon ignition or sudden impact according to the balanced equation: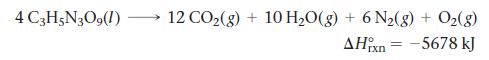
Calculate the standard enthalpy of formation (ΔH°f) for nitroglycerin.
Transcribed Image Text:
4 C3H5N3O9 (1) 12 CO₂(g) + 10 H₂O(g) + 6 N₂(g) + O₂(g) AHixn -5678 kJ =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To calculate the standard enthalpy of formation Hf for nitroglycerin we can use the following equati...View the full answer

Answered By

Akash Goel
I am in the teaching field since 2008 when i was enrolled myself in chartered accountants course
Since then i have an experience of teaching of class XI, XII, BCOM, MCOM, MBA, CA CPT.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation (H f) for nitroglycerin. The explosive nitroglycerin (C3H5N3O9) decomposes rapidly upon ignition or sudden impact according to the following balanced...
-
Nitroglycerin, one of the most commonly used explosives, has the following structure The decomposition reaction is The explosive action is the result of the heat released and the large increase in...
-
The standard enthalpy change (H° for the thermal decomposition of silver nitrate according to the following equation is 178.67 kJ: The standard enthalpy of formation of AgNO3(s) is 2123.02...
-
George operates a business that generated adjusted gross income of $250,000 and taxable income of $170,000 this year (before the domestic production activities deduction). Included in income was...
-
Otto Company maintains a petty cash fund for small expenditures. These transactions occurred during the month of August. Aug. 1 Established the petty cash fund by writing a check on Central Bank for...
-
A small stock dividend a. decreases common stock. b. has no effect on total equity. c. increases Retained Earnings. d. Items a, b, and c are correct.
-
Howcould Collegiate Apparels president or a companys board of directors use the budget to influence the future direction of the firm? Would participative budgeting be effective for Collegiate Apparel...
-
Rossco is considering the purchase of a new computer with the following estimated costs: initial systems design, $54,000; hardware, $74,000; software, $35,000, one-time initial training, $11,000;...
-
A trucker driver can expect to make $US50,456 per year in the US. Assuming that this driver works 70 hours per week and 52 weeks per year, what is the equivalent salary in Canadian dollars per hour?...
-
On November 10, 2020, Singh Electronics began to buy and resell scanners for $55 each. Singh uses the perpetual system to account for inventories. The scanners are covered under a warranty that...
-
Determine the mass of CO 2 produced by burning enough of each fuel to produce 1.00 * 10 2 kJ of heat. Which fuel contributes least to global warming per kJ of heat produced? a. CH.(g) + 2 O2(g) b....
-
Ethanol (C 2 H 5 OH) can be made from the fermentation of crops and has been used as a fuel additive to gasoline. Write a balanced equation for the combustion of ethanol and calculate H rxn .
-
In a random sample of 472 owners of small businesses that had gone into bankruptcy, 352 reported conducting no marketing studies prior to opening the business. Test the hypothesis that at most 70% of...
-
1. (5 pts) Given y[n]= 2y[n-1] and y[0]=2, Write MATLAB code to calculate and plot y for 0
-
F ( t ) = t 4 + 1 8 t 2 + 8 1 2 , g ( t ) = ( t + 3 ) / 3 ; find ( f o g ) ( 9 )
-
How did they calculate allocated cost FLIGHT A FLIGHT 350 615 FLIGHT 3 1 Go GALS 20 G EXISTING SCHEME, DETERMINE THE OVE OR FLIGHTS A, B, AND C. 2 ED AT 7.00 PER K1.00 OF PILOT SALAF TOTAL NON-SALARY...
-
High Tech ManufacturingInc., incurred total indirect manufacturing labor costs of $540,000. The company is labor-intensive. Total labor hours during the period were 5,000. Using qualitativeanalysis,...
-
Start with AS/AD and IS/MP in full employment equilibrium. Assume the is a massive positive aggregate demand shock. How would this affect AS/AD and IS/MP and prices and output relative to the full...
-
Refer to Exercise 4-45. Suppose that the standard deviations of the values of lost time due to accidents before and after use of the OSHA program are unknown but are assumed to be equal. The first...
-
Find the velocity, acceleration, and speed of a particle with the given position function. r(t) = (t 2 , sin t - t cos t, cos t + t sin t), t > 0
-
Show that the presence of a C 2 axis and a mirror plane perpendicular to the rotation axis imply the presence of a center of inversion.
-
Decompose the following reducible representation into irreducible representations of the C 3v group: 30v 2C3 1 5 2.
-
Assume that a central atom in a molecule has ligands with C 4v symmetry. Decide by evaluating the appropriate transition dipole element if the transition p x p z is allowed with the electric field...
-
In 1975 the price of a new house was $48,273. In 2020 the price of a new house is $185,524. How much has the price of housing increased over the entire time period in percentage terms? State your...
-
CHOP Inc., which makes only one product, Yester, has the following information available for the coming year. CHOP expects sales to be 27,000 units at $32 per unit. The current inventory of Yester is...
-
Huron Company produces a commercial cleaning compound known as Zoom. The direct materials and direct labor standards for one unit of Zoom are given below: Standard Quantity or Standard Hours Standard...
The Individual Investors Guide To Computerized Investing 11th Edition - ISBN: 0942641515 - Free Book

Study smarter with the SolutionInn App


