The heating value of combustible fuels is evaluated based on the quantities known as the higher heating
Question:
The heating value of combustible fuels is evaluated based on the quantities known as the higher heating value (HHV) and the lower heating value (LHV). The HHV has a higher absolute value and assumes that the water produced in the combustion reaction is formed in the liquid state. The LHV has a lower absolute value and assumes that the water produced in the combustion reaction is formed in the gaseous state. The LHV is therefore the sum of the HHV (which is negative) and the heat of vaporization of water for the number of moles of water formed in the reaction (which is positive). The table lists the enthalpy of combustion—which is equivalent to the HHV—for several closely related hydrocarbons.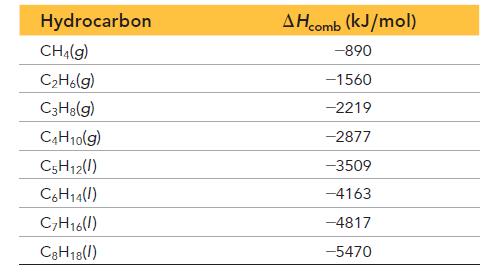
Use the information in the table to answer the following questions.
a. Write two balanced equations for the combustion of C3H8; one assuming the formation of liquid water and the other assuming the formation of gaseous water.
b. Given that the heat of vaporization of water is 44.0 kJ/mol, what is ΔHrxn for each reaction in part a? Which quantity is the HHV? The LHV?
c. When propane is used to cook in an outdoor grill, is the amount of heat released the HHV or the LHV? What amount of heat is released upon combustion of 1.00 kg of propane in an outdoor grill?
d. For each CH2 unit added to a hydrocarbon, what is the average increase in the absolute value of ΔHcomb?
Step by Step Answer:






