The kinetics of this reaction were studied as a function of temperature. a. Determine the activation energy
Question:
The kinetics of this reaction were studied as a function of temperature.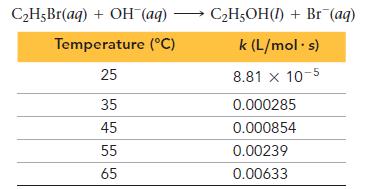
a. Determine the activation energy and frequency factor for the reaction.
b. Determine the rate constant at 15 °C.
c. If a reaction mixture is 0.155 M in C2H5Br and 0.250 M in OH-, what is the initial rate of the reaction at 75 °C?
Transcribed Image Text:
C₂H5Br(aq) + OH-(aq) Temperature (°C) 25 35 45 55 65 C2₂H5OH(1) + Br (aq) k (L/mol s) 8.81 x 10-5 0.000285 0.000854 0.00239 0.00633
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a Ea 895 kJm...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A second-order reaction has a rate constant of 8.7 104/(Ms) at 30oC. At 40oC, the rate constant is 1.8 103/(Ms). What are the activation energy and frequency factor for this reaction? Predict the...
-
For the reaction I (aq) + OCl (aq) OI (aq) + Cl (aq) occurring in aqueous solution, the following mechanism has been proposed: a. Derive the rate law expression for this reaction based on this...
-
The bond strength when mounting an integrated circuit on a metalized glass substrate was studied as a function of factor A = adhesive type, factor B = curve time, and factor C = conductor material...
-
Determine whether the given set of matrices under the specified operation, matrix addition or multiplication, is a group. Recall that a diagonal matrix is a square matrix whose only nonzero entries...
-
Refer to Exercise 5-6. Required: 1. Prepare journal entries for the August transactions. 2. Calculate the ending balances of each of the inventory accounts as of August 31.
-
A supermarket wholesaler sells over 40,000 product lines to retailers who visit the store. It has $45,000 \mathrm{~m}^{3}$ of general storage including $100 \mathrm{~m}^{3}$ of cold storage. General...
-
(Appendix) Under what conditions is goodwill recognized on the balance sheet of the par ent?
-
CVP analysis, changing revenues and costs Sunshine Travel Agency specializes in flights between Toronto and Jamaica. It books passengers on Canadian Air. Sunshines fixed costs are $22,000 per month....
-
Jordan Corporation is considering a new product that will be very popular for a couple of years and then slowly lose its commercial appeal. Sales are projected to be $90,000 in year one, $100,000 in...
-
The evaporation of a 120-nm film of n-pentane from a single crystal of aluminum oxide is zero order with a rate constant of 1.92 * 10 13 molecules/cm 2 s at 120 K. a. If the initial surface coverage...
-
The desorption (leaving of the surface) of a single molecular layer of n-butane from a single crystal of aluminum oxide is found to be first order with a rate constant of 0.128/s at 150 K. a. What is...
-
Firm D has net income of 27,900 sales of $930,000 and average total assets of 465,000 Calculate the firms margin, turnover, and ROI
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Discuss the principles of geotechnical engineering in slope stability analysis. How can engineers assess slope stability, mitigate landslide risks, and design effective stabilization measures to...
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Briefly explain what spatial autocorrelation means and what method can be used to measure it
-
Discuss the theoretical implications of adopting biodegradable materials in civil engineering for reducing environmental impact and enhancing sustainability.
-
For each of the following measures, determine whether it is monotone, anti-monotone, or non-monotone (i.e., neither monotone nor anti-monotone). Example: Support, s = Ï(X)/|T| is anti-monotone...
-
-4 1 9. Let A = Find A-1, (A") and verify that (A")= (A-1)".
-
When a beam of white light passes perpendicularly through a flat pane of glass, it is not dispersed into a spectrum. Why not?
-
Describe the image the lens of the eye forms on the retina.
-
The candle of Exercise 53 is 30 cm from the lens. Answer the same questions for this situation.
-
5. The cost of retained earnings the required rate of If a firm cannot invest retained earnings to earn a rate of return return on retained earnings, it should return those funds to its st less than...
-
How much will be the loss from bad debts under new credit terms of 3/10 net, if the cost of capital is 15% and the unpaid accounts are written off after 60 days?
-
You need to accumulate $10,000. To do so, you plan to make deposits of $1,100 per year, with the first payment being made a year from today, in a bank account that pays 7 percent annual interest....

Study smarter with the SolutionInn App


