The phase diagram for sulfur is shown here. The rhombic and monoclinic states are two solid states
Question:
The phase diagram for sulfur is shown here. The rhombic and monoclinic states are two solid states with different structures.
a. Below what pressure does solid sulfur sublime?
b. Which of the two solid states of sulfur is more dense?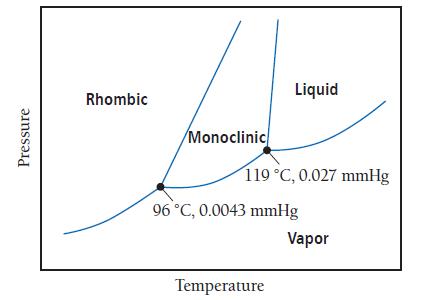
Transcribed Image Text:
Pressure Rhombic Monoclinic Liquid 119 °C, 0.027 mmHg 96 °C, 0.0043 mmHg Temperature Vapor
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
a 00...View the full answer

Answered By

Mary Njunu
I posses Vast, diversified knowledge and excellent grammar as a result of working in ACADEMIC WRITING for more than 5 years. I deliver work in various disciplines with assurance of quality work. I purpose at meeting the clients’ expectations precisely. Let’s work together for the best and phenomenal grades.
4.90+
929+ Reviews
2557+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the accompanying phase diagram for sulfur to answer the following questions. (The phase diagram is not to scale.) a. How many triple points are in the phase diagram? b. What phases are in...
-
Why are there no points in the phase diagram for sulfur in Figure 8.11 that show rhombic and monoclinic solid phases in equilibrium with liquid and gaseous sulfur? Figure 8.11 Liquid Monoclinic-...
-
Look at the phase diagram for sulfur in Figure 11.12, bottom. What states of sulfur would you expect to see at each of the triple points? What solid phase would you expect to freeze out if you cooled...
-
The combined sewer system in city ABC is comprised of two parallel interceptors referred to as "North" and "South" lines. The southern line is connected to a newly built wastewater treatment plant....
-
The Federal Crop Insurance Corporation (FCIC) was created as a wholly government-owned corporation to insure wheat producers against unavoidable crop failure. As required by law, the FCIC published...
-
Company A has 500 employees and Company B has 800 employees. Would the analyst working with Company B need to interview more employees than the analyst working with Company A? Why or why not?
-
What are the accounting standards (A.S.) formulated by ASB in India and the corresponding International accounting standards (I.A.S.)?
-
Telly, age 38, has a $140,000 IRA with Blue Mutual Fund. He has read good things about the management of Green Mutual Fund, so he opens a Green Fund IRA. Telly asked for and received his balance from...
-
Part D... Total 5 Points; Suggested Time Limit: 25 Minutes Maximum Our financial accounting/reporting model has been criticized as being, primarily, a historic cost based and transaction based model....
-
Sandra?s Purse Boutique has the following transactions related to its top-selling Gucci purse for the month of October. Required: 1. Calculate ending inventory and cost of goods sold at October 31,...
-
The high-pressure phase diagram of ice is shown here. Notice that, under high pressure, ice can exist in several different solid forms. What three forms of ice are present at the triple point marked...
-
Show how the fluorite structure accommodates a cation-toanion ratio of 1:2.
-
What is the correct unit for intensity? A. J m 2 B. J s 1 C. W m 2 D. W m 2
-
The free-rolling ramp has a mass of 40 kg. A 10-kg crate is released from rest at A and slides down 3.5 m to point B. (Figure 1) If the surface of the ramp is smooth, determine the ramp's speed when...
-
In computer forensics, you are often confronted with a different organisation of multi-byte values. Two common ways to order the bytes are big-endian (e.g. SUN Sparc, Apple) and little-endian (e.g....
-
1-Define electric fields and how it helps us understand electricity. 2-Electric fields are represented as a physical effect of a configuration of charges that is created by the attraction of electric...
-
Product A will be cleaned out. The product has a standard daily dose of 10mg and the batch size is 200kg. The next product B has standard a daily does of 250mg and the batch size is 50kg. Both A and...
-
Given a physical memory size of 290k, simulate using multiple partition allocation (MVT). Assume that the OS occupies the first 40k. Answer the ff. questions below: OS 40k-1 Process ID Arrival Time...
-
What reagent will react by addition to what unsaturated hydrocarbon to form each of the following compounds? a. CH3CHBrCHBrCH3 b. (CH3)2CHOSO3H c. (CH3)3COH d. e. CH3CH=CHCH2Cl f. CH3CH2CCl2CCl2CH3...
-
Write a declaration for each of the following: a. A line that extends from point (60, 100) to point (30, 90) b. A rectangle that is 20 pixels wide, 100 pixels high, and has its upper-left corner at...
-
It is believed that penicillin antibiotics are biosynthesized from amino acid precursors. Identify the two amino acids that are most likely utilized during the biosynthesis of penicillin antibiotics:...
-
Draw the s-cis conformation of the dipeptide Phe-Phe and identify the source of steric hindrance.
-
Using a bond-line structure, show the tetrapeptide obtained when two molecules of Cys-Phe are joined by a disulfide bridge.
-
Example 3-1 In 20--, the annual salaries paid each of the officers of Abrew, Inc., follow. The officers are paid semimonthly on the 15th and the last day of the month. Compute the FICA taxes to be...
-
Jaybird Company operates in a highly competitive market where the market price for its product is $120 per unit. Jaybird desires a 30% profit per unit. Jaybird expects to sell 5,000 units. Additional...
-
2.2. You have a stock in the three-period binomial model such that So = 4, S.(H) = 8, S (T) = 2, and r=0.25. (c) Work out the value tree for a forward contract with delivery time 3 and delivery price...

Study smarter with the SolutionInn App


