Why are there no points in the phase diagram for sulfur in Figure 8.11 that show rhombic
Question:
Figure 8.11
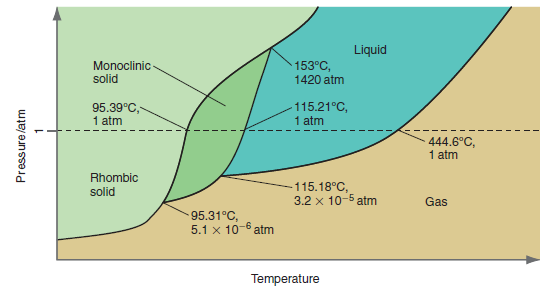
Transcribed Image Text:
Liquid Monoclinic- solid 153°C, 1420 atm 95.39°C, 1 atm 115.21°C, 1 atm 444.6°C, 1 atm Rhombic -115.18°C, 3.2 x 10-5 atm solid Gas 95.31°C, 5.1 x 10-6 atm Temperature Pressure/atm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (19 reviews)
According to the ...View the full answer

Answered By

Ashok Kumar Malhotra
Chartered Accountant - Accounting and Management Accounting for 15 years.
QuickBooks Online - Certified ProAdvisor (Advance - QuickBooks Online for 3 years.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The phase diagram of NH 3 can be characterized by the following information. The normal melting and boiling temperatures are 195.2 and 239.82 K, respectively; the triple point pressure and...
-
A PT phase diagram for potassium is shown below. a. Which phase has the higher density, the fcc or the bcc phase? Explain your answer. b. Indicate the range of P and T in the phase diagram for which...
-
Why are there no sp4 or sp5 hybrid orbitals?
-
The contingency table shown relates happiness and gender for the 2012 GSS. a. Identify the response variable and the explanatory variable. b. Construct a table or graph showing the conditional...
-
1. Draw the appropriate network diagram. 2. Find the Critical Path and the project completion time. 3. Find the Probability that the project will take more than 50 days to complete. Please use excel...
-
For a new type of biofuel, scientists estimate that it takes A = f(g) gallons of gasoline to produce the raw materials to generate g gallons of biofuel. Assume the biofuel is equal in efficiency to...
-
What is the major difference between the MRP push systems and the pull system? LO,1
-
A factory costs $800,000. You reckon that it will produce an inflow after operating costs of $170,000 a year for 10 years. If the opportunity cost of capital is 14 percent, what is the net present...
-
Troy Engines, Ltd., manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
Consider the following Regular Expression: a(bb)*bc Draw a DFA for the above RE. Determine the language accepted by this automaton Implement this DFA in C/C++/Java. Your implementation should...
-
Calculate the degree of dissociation of N 2 O 4 in the reaction N 2 O 4 (g) 2NO 2 (g) at 300. K and a total pressure of 1.50 bar. Do you expect the degree of dissociation to increase or decrease as...
-
Predict which of the following substrates will undergo an E1 reaction more quickly. Explain your choice. Br Br or
-
In Example 2, what percentage of the test scores are between 350 and 650? Data from Example 2 The scores on a certain achievement test are normally distributed with a mean of 500 and a standard...
-
Noeleen AutoMall, Ltd. recently completed an initial public offering(IPO) for$23,000,000 by listing its common shares on the New York Stock Exchange. Prior to itsIPO, Noeleen was a privately held...
-
Process Costing- increased units, FIFO method Answer in good form 25 26 Illustrative Problem-Cost of Production Report using Treatment by Neglect Dept 1-100% of materials are added at the beginning....
-
Write a C++ program that prompts the user to enter a letter and encrypt it using the following method: if the letter is an upper-case letter the program replaces the letter by the 7th letter in the...
-
Turn this information into an excel sheets with the excel formulas being shown P12.4 (LO 1) (Payroll Tax Entries) The following is a payroll sheet for Otis Imports for the month of September 2025....
-
What is the major factor preventing women of the Indian diaspora from engaging in elaborate rituals? Group of answer choices Lack of motivation or devotion Because rituals are tied to a place and...
-
In Exercises 23 through 34, determine the critical numbers of the given function and classify each critical point as a relative maximum, a relative minimum, or neither. g(x) = (x 1) 5
-
Determine the resultant moment produced by the forces about point O. 0.25 m 0.125 m, 0 0.3 m- 60 F = 500 N F = 600 N
-
A gas obeying the equation of state p(V-nb) = nRT is subjected to a Joule- Thomson expansion. Will the temperature increase, decrease, or remain the same?
-
Rearrange the van der Waals equation of state to give an expression for T as a function of p and V (with n constant). Calculate (T/p)v and confirm that (T/p)v= l/(p/D")v. Go on to confirm Euler's...
-
On a cold, dry morning after a frost, the temperature was -5C and the partial pressure of water in the atmosphere fell to 0.30 kPa. Will the frost sublime? What partial pressure of water would ensure...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


