The vapor pressure of a 1 M ionic solution is different from the vapor pressure of a
Question:
The vapor pressure of a 1 M ionic solution is different from the vapor pressure of a 1 M nonelectrolyte solution. In both cases, the solute is nonvolatile. Which set of diagrams best represents the differences between the two solutions and their vapors?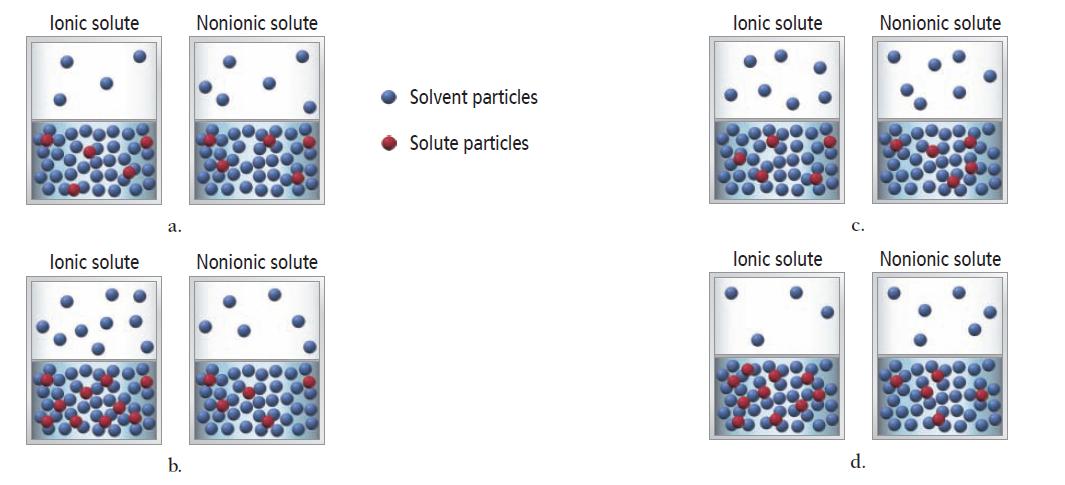
Transcribed Image Text:
lonic solute lonic solute a. b. Nonionic solute Nonionic solute Solvent particles Solute particles lonic solute lonic solute C. d. Nonionic solute Nonionic solute
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The correct set of diagrams that best represents the differences between the 1 M ionic solution and the 1 M nonelectrolyte solution and their vapors i...View the full answer

Answered By

CHARLES AMBILA
I am an experienced tutor with more than 7 years of experience. I have helped thousands of students pursue their academic goals. My primary objective as a tutor is to ensure that students have easy time handling their academic tasks.
5.00+
109+ Reviews
323+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The vapor pressure of various substances can be determined using effusion. In this process, the material of interest is placed in an oven (referred to as a Knudsen cell) and the mass of material lost...
-
What is the effect of a nonvolatile solute on the vapor pressure of a liquid? Why is the vapor pressure of a solution different from the vapor pressure of the pure liquid solvent?
-
What is the vapor pressure of a solution in which the mole fraction of the solute is 0.200 and the vapor pressure of the pure solvent is 100.0 torr? (Assume a single nonvolatile, nonelectrolyte...
-
Integrate using an appropriate formula For each problem, state the formula number, u and du, identify any constants (if appropriate), and show any constant "adjustments" / "Multiply by 1" if needed....
-
The LeVitre and Swezey Credit Union maintains separate bank accounts for each of its 20,000 customers. Three major files are the customer master file, the transaction file of deposits and withdrawal...
-
A company issued five-year, 5% bonds with a par value of $100,000. The company received $95,735 for the bonds. Using the straight-line method, the companys interest expense for the first semiannual...
-
Describe three ratios that relate a firm's stock price to its earnings, cash flow, and book value per share, and write out their equations. AppendixLO1
-
Accounting, Analysis, and Principles Diversified Products, Inc. operates in several lines of business, including the construction and real estate industries. While the majority of its revenues are...
-
What would be the right answers for the ones that are marked red ? GL1201 - Based on Exercise 12-11 LO P2, P3, A1 Use the following financial statements and additional information. 2018 FIELDS INC....
-
Geographers use remote-sensing data from satellite pictures to identify urban land cover as either grassland, commercial, or residential. In Geographical Analysis (Oct. 2006), researchers from...
-
If each substance listed here costs the same amount per kilogram, which would be most cost-effective as a way to lower the freezing point of water? (Assume complete dissociation for all ionic...
-
A power plant built on a river uses river water as a coolant. The water is warmed as it is used in heat exchangers within the plant. Should the warm water be immediately cycled back into the river?...
-
(a) Consider the following nuclear process, in which a proton is removed from an oxygen nucleus: Find the energy required for this process to occur. (b) Now consider a process in which a neutron is...
-
Compare Figures 1-2 and 1-12. How do they differ? How are they similar? Explain how Figure 1-12 conveys the idea of speed in development. Figures 1-2 Figures 1-12 Maintenance Planning Implementation...
-
With a neat sketch explain the working of pressure-velocity compounding of impulse steam turbine.
-
The adjusted trial balance for Barry Moving Service as of December 31 is as follows: Required a. Prepare the closing entries at December 31 directly to Retained Earnings in general journal form. b....
-
Consider a piston with an orifice in a cylinder filled with a fluid of viscosity \(\mu\) as shown in Fig. 1.106. As the piston moves in the cylinder, the fluid flows through the orifice, giving rise...
-
Add a function to SmallWorld that computes the global clustering coefficient of a graph. The global clustering coefficient is the conditional probability that two random vertices that are neighbors...
-
Phosphorus atoms are to be diffused into a silicon wafer using both predeposition and drive-in heat treatments; the background concentration of P in this silicon material is known to be 5 1019...
-
The Zwatch Company manufactures trendy, high-quality moderately priced watches. As Zwatch's senior financial analyst, you are asked to recommend a method of inventory costing. The CFO will use your...
-
Starting with Eq. (3.32), prove that the energy densities of the electric and magnet fields are equal (u E = u B ) for an electromagnetic wave. (3.32) UB 2o
-
Prove that the irradiance of a harmonic EM wave in vacuum is given by and then determine the average rate at which energy is transported per unit area by a plane wave having an amplitude of 15.0 V/m....
-
A nearly cylindrical laserbeam impinges normally on a perfectly absorbing surface. The irradiance of the beam (assuming it to be uniform over its cross section) is 40 W/cm 2 . If the diameter of the...
-
true- false statement (c) Cost-based accounting is conservative
-
C. Inventory Revaluation Outdoor Recreation has the following three trailers in stock at the end of the year: Model #1103 #1204 #1305 Original cost 5,500 7,200 9,000 Expected sales price 5,700 8,500...
-
true- false statement (8) Unanimity implies that shareholders have no incentive to use their voting rights. (1) With corporate income tax, retention dominates dividends

Study smarter with the SolutionInn App


