The vapor pressure of dichloromethane is measured as a function of temperature, and the results are tabulated.
Question:
The vapor pressure of dichloromethane is measured as a function of temperature, and the results are tabulated. From the results, determine the heat of vaporization of dichloromethane.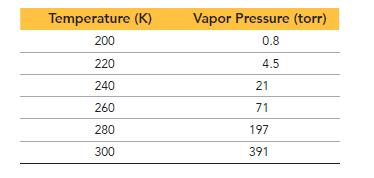
Transcribed Image Text:
Temperature (K) 200 220 240 260 280 300 Vapor Pressure (torr) 0.8 4.5 21 71 197 391
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To find the heat of vaporization use an Excel sprea...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. The following data have been reported for the vapor pressure of ethanol as a function of temperature. Use these data to calculate the heat of vaporization of ethanol at 17.33C. b. Ackermann and...
-
The Dew Point The vapor pressure of water (see Problem 18.88) decreases as the temperature decreases. If the amount of water vapor in the air is kept constant as the air is cooled, a temperature is...
-
We have learned that the enthalpy of vaporization of a liquid is generally a function of temperature. If we wish to take this temperature variation into account, we cannot use the ClausiusClapeyron...
-
Terminology Key: Key Word List Describe Explain What to do In bulleted, numbered or paragraph form, provide a number of consecutive items-if paragraph form, use commas (,) to separate items In...
-
Why is interest paid on amounts borrowed from banks and other lenders considered to be an operating activity while the amounts borrowed are financing activities?
-
Which of the following is NOT an argument for a zero rate of inflation? a. It eliminates distortions from a non-indexed tax code. b. It encourages people to hold a greater quantity of money. c. It...
-
What are the key performance drivers re efficiency? lop4
-
Kunkel Company makes two products and uses a traditional costing system in which a single plantwide predetermined overhead rate is computed based on direct labor-hours. Data for the two products for...
-
QS 16-3 Indirect: Computing cash flows from operations LO P2 The list includes all balance sheet accounts related to cash from operating activities Net Income Depreciation expense Accounts receivable...
-
When did the rapid development of the management science discipline begin?
-
An electrostatic potential map for acetonitrile (CH 3 CN), which is polar, is shown here: From this map, determine the geometry for how two acetonitrile molecules would interact with each other....
-
What are the main properties of liquids (in contrast to gases and solids)?
-
Make an argument for Marine Midland that the bank took reasonable care of the collateral.
-
When CH4(g) reacts with O2(g) to form CO2(g) and H2O(g), 192 kcal of energy are evolved for each mole of CH4(g) that reacts. Write a balanced equation for the reaction with an energy term in kcal as...
-
Ben Rogers, Judy Wilkinson, and Henry Walker were the partnership dentists. Ben Rogers became insolvent because of real estate investments. So, Judy Wilkinson and Henry Walker had to then pay the...
-
A random sample of 10 subjects have weights with a standard deviation of 10.8148 kg. What is the variance of their weights? Be sure to include the appropriate units with the result.
-
of stion 1. Harmonic Test II. Root Test III. Ratio Test Consider the series 8 = 72 Which one of the following tests can be used to determine whether it is convergent or divergent? IV. Integral Test...
-
Pharaoh company obtains $44,800 in cash by signing a 7%, 6 month, $44,800 note payable to First Bank on July 1. Pharoah's fiscal year ends on September 30. What information should be reported for the...
-
Write the structure obtained when electrons move as indicated by the curved arrows in the following structure: Does each atom in the resulting structure have a complete valence shell of electrons?...
-
Factor and simplify, if possible. Check your result using a graphing calculator. 3 cot 2 + 6 cot + 3
-
When the temperature is at 30°C, the A-36 steel pipe fits snugly between the two fuel tanks. When fuel flows through the pipe, the temperatures at ends A and B rise to 130°C and 80°C,...
-
The bar has a cross-sectional area A, length L, modulus of elasticity E, and coefficient of thermal expansion a. The temperature of the bar changes uniformly along its length from T A at A to T B at...
-
The device is used to measure a change in temperature. Bars AB and CD are made of A-36 steel and 2014-T6 aluminum alloy, respectively. When the temperature is at 75°F, ACE is in the horizontal...
-
Suppose an investment is equally likely to have a 42% return or a -20% return. The total volatility of returns is closest to: Select one: a. 9.61% b. 43.84% c. 21.92% d. 31.00%
-
Project DEF Initial End-of-Year Investment Cash Flows for years 1-3, respectively $32,000 $20,000 30,000 17,000 WACC = 17% What is the Profitability Index? (Please round to the nearest hundredth and...
-
A company owes $100 to be paid at times 2, 4, and 6. The company plans to meet the obligation with an investment program that produces asset cash flows of A1 at time 1 and A5 at time 5 using...

Study smarter with the SolutionInn App


