These reactions are important in catalytic converters in automobiles. Calculate G for each at 298 K. Predict
Question:
These reactions are important in catalytic converters in automobiles. Calculate ΔG° for each at 298 K. Predict the effect of increasing temperature on the magnitude of ΔG°.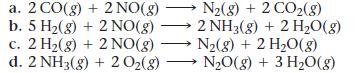
Transcribed Image Text:
a. 2 CO(g) + 2NO(g) b. 5 H₂(g) + 2NO(g) c. 2 H₂(g) + 2NO(g) d. 2 NH3(g) + 2 O₂(g) N₂(g) + 2 CO₂(8) 2 NH3(g) + 2 H₂O(g) N₂(g) + 2 H₂O(g) N₂O(g) + 3 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a G 6896 kJ G becomes less ne...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
2. A centrifugal pump operating at 1000 rpm is delivering 18.2 m/hr of water at 10.3 bar and requires 6 kW of shaft power at the best efficiency point. Determine (a) the flow rate, (b) discharge...
-
a. Predict the effect of increasing the temperature on the reaction: H 2 (g) + CO 2 (g) H 2 O(g) + CO(g) H r = +41.2 kJ mol 1 b. In the reaction Ag 2 CO 3 (s) Ag 2 O(s) + CO 2 (g) increasing the...
-
Amoeba Propagation An amoeba propagates by simple division; each split takes 3 minutes to complete. When such an amoeba is put into a glass container with a nutrient fluid, the container is full of...
-
Briefly explain the DHCP lease process. What packets are sent and when are they sent? (5 marks) Question 2 any three advantages of IPv6 over IPv4? How many classes are there in IPv4 and what is the...
-
Flagstaff Enterprises makes flagpoles. Dan Dalripple, the company's new controller, can find only the following partial information for the past two months: The current year's predetermined overhead...
-
1. Develop a model that will allow Applecore to maximize the number of customers reached for a budget of $10,000 for one week of promotion. 2. Solve the model. What is the maximum number of customers...
-
The financial statements of Freezeqwik Ltd, a distributor of frozen foods, for the year ended 31 December last year are: Income statement for the year ended 31 December last year 000 000 Sales...
-
Suppose in its 2014 annual report, McDonalds Corporation reports beginning total assets of $28.46 billion; ending total assets of $30.22 billion; net sales of $22.74 billion; and net income of $4.55...
-
Dubberty Corporation's cost formula for its manufacturing overhead is $31,700 per month plus $54 per machine hour. For the month of March, the company planned for activity of 8,120 machine-hours, but...
-
All the oxides of nitrogen have positive values of G f at 298 K, but only one common oxide of nitrogen has a positive S f . Identify that oxide of nitrogen without reference to thermodynamic data and...
-
Consider this reaction occurring at 298 K: a. Show that the reaction is not spontaneous under standard conditions by calculating G rx n . b. If BaCO 3 is placed in an evacuated flask, what is the...
-
Tetrahydrofuran (a) and water (b) separate into two liquid phases at 1 bar and 50C. Determine the composition of each liquid phase. The following three-suffi x Margules parameters have been obtained...
-
Use the Comprehensive Annual Financial Report for the Village of Arlington Heights (please look up this content) for the year ended December 31, 2018, to answer questions 8-20. All questions are on...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Two wires lie perpendicular to the plane of the screen and carry equal magnitudes of electric current in the directions shown. Point P is equidistant from the two wires. The distance between each of...
-
Your firm has recently been appointed as auditors of Kentronics Ltd , a large company which markets sophisticated electronic equipment for heavy industry as well as the mining equipment industry. The...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Jay Oullette, CEO of Bumper to Bumper, Inc., anticipates that his company's year-end balance sheet will show current assets of $180,000 and current liabilities of $100,000. Mr. Oullette has asked...
-
Will the prediction interval always be wider than the estimation interval for the same value of the independent variable? Briefly explain.
-
In Section 11.3, we discussed the total mechanical energy of a mass-on-a-spring oscillator. The result in Equation 11.21 shows that the total energy is proportional to the square of the amplitude....
-
Use energy considerations to derive the oscillation frequency for a mass-on-a-spring oscillator. The maximum potential energy stored in the spring must be equal to the maximum kinetic energy of the...
-
Derive the frequency of oscillation of a torsional oscillator, Equation 11.20. Consider how the restoring force depends on the twist angle and use Newton?s second law for rotational motion (? = I?)....
-
The following is part of the computer output from a regression of monthly returns on Waterworks stock against the S&P 5 0 0 index. A hedge fund manager believes that Waterworks is underpriced, with...
-
Doisneau 25-year bonds have an annual coupon interest of 8 percent, make interest payments on a semiannual basis, and have a $1,000 par value. If the bonds are trading with a market's required yield...
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...

Study smarter with the SolutionInn App


