This table displays the vapor pressure of nitrogen at several different temperatures. Use the data to determine
Question:
This table displays the vapor pressure of nitrogen at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of nitrogen.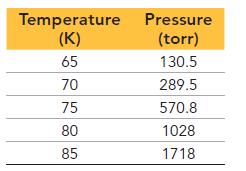
Transcribed Image Text:
Temperature Pressure (K) (torr) 65 130.5 289.5 570.8 1028 1718 70 75 80 85
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
To determine the heat of vaporization and normal boiling point of nitrogen we can use the ClausiusCl...View the full answer

Answered By

Shristi Singh
A freshman year metallurgy and material science student in India.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
This table displays the vapor pressure of ammonia at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of ammonia. Temperature Pressure (K)...
-
Procurement of commodities is a critical activity for a company's survival, particularly in the manufacturing industry. It is essential in the industry because it standardizes the procurement of...
-
Suppose the vapor pressure of a substance is measured at two different temperatures. (a) By using the Clausius-Clapeyron equation (Equation 11.1) derive the following relationship between the vapor...
-
The management of a New York area investment firm wants to find out about the investment needs of its existing customers, for which it has an extensively detailed list, as a function of their...
-
EZRest Motel is a motel with 216 rooms located in the center of a large city in State Y. It is readily accessible from two interstate highways and three major State highways. The motel solicits...
-
Apply the criterion for inherent instability of a single liquid phase to determine the composition range at which the system in Example 8.19 will spontaneously split into two phases. Example 8.19...
-
Another family of products for Mesa Table Company has the following projected monthly demand: There are currently 30 workers each capable of producing 50 tables per month. They have a no-inventory...
-
Johnstone Inc. began operations in January 2011 and reported the following results for each of its 3 years of operations. 2011 $260,000 net loss 2012 $40,000 net loss 2013 $700,000 net income At...
-
5 In an assembly shop of a Motor Cycle Factory 4 workers A, B, C and D work together as a team and are paid on group piece rate. They also work individually on day rate jobs. In a 44 hour week the...
-
E-Z Loan, Co., makes loans to high-risk borrowers. E-Z borrows from its bank and then lends money to people with bad credit. The bank requires E-Z Loan to submit quarterly financial statements in...
-
Ethanol has a heat of vaporization of 38.56 kJ/mol and a normal boiling point of 78.4 C. What is the vapor pressure of ethanol at 15 C?
-
Suppose that 1.15 g of rubbing alcohol (C 3 H 8 O) evaporates from a 65.0-g aluminum block. If the aluminum block is initially at 25 C, what is the final temperature of the block after the...
-
Develop sales material that describes your services, the benefits they provide, and why your target market should buy those products. Try your sales pitch on friends and family.
-
Describe the overlap between business law and criminal law. Be sure to use examples, and discuss at least three (3) different domains where these two areas of law overlap.
-
Problem 3-12 Linden Corp. has a 10% market share in its industry. Below are income statements ($M) for Linden and for the industry. Linden Industry Sales $6,200 $66,000 Cost of Goods Sold 3,100...
-
Economic order quantity; order cost; carrying cost Starr Company predicts that it will use 360,000 units of material during the year. The material is expected to cost $5 per unit. Starr anticipates...
-
Requirement 1. Prepare a horizontal analysis of the comparative income statement of McCormick Designs, Inc. Round percentage changes to one decimal place. (Round the percentages to one decimal place,...
-
GAAP looks to compare entities with an apples-to-apples valuation so that the entities can be viewed by interested parties to compare financial values and how effectively and efficiently they use...
-
Write an equation for the reaction of a sodium acetylide with water.
-
The diagram shows the two forces acting on a small object. Which of the following is the resultant force on the object? A. 8 N downwards B. 8 N upwards C. 2 N downwards D. 2 N upwards 3 N 5 N
-
Draw the structure of the protected amino acid that must be anchored to the solid support in order to use a Merrifield synthesis to prepare leucine enkephalin. (N terminus) Try-Gly-Gly-Phe-Leu (C...
-
Draw all four possible dipeptides that are obtained when a mixture of l-phenylalanine and l alanine is treated with DCC.
-
Show all steps necessary to make the dipeptide Phe-Ala from l-phenylalanine and l-alanine.
-
1) issued stock for $72,000 2) borrowed $41,000 from its bank 3) provided consulting services for $71,000 cash 4) paid back $31,000 of the bank loan 5) paid rent expense for $17,000 6) purchased...
-
Centurion Co. had the following accounts and balances at December 31: Account Cash Accounts Receivable Prepaid Insurance Supplies Accounts Payable T. Happy, Capital Service Revenue Salaries Expense...
-
Gretchen invests 6200 dollars in a mutual fund on January 1. On March 1, she learns that her fund balance is 3800 dollars, and she then withdraws 1500 dollars. On August 1, her fund balance is 7800...

Study smarter with the SolutionInn App


