This table displays the vapor pressure of ammonia at several different temperatures. Use the data to determine
Question:
This table displays the vapor pressure of ammonia at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of ammonia.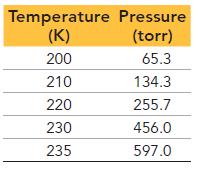
Transcribed Image Text:
Temperature Pressure (K) (torr) 200 65.3 210 134.3 220 255.7 230 456.0 235 597.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Hvap ...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
This table displays the vapor pressure of nitrogen at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of nitrogen. Temperature Pressure (K)...
-
a) Sputtering is a very important physical process in plasma engineering of thin films and coatings. In one or two paragraphs describe what sputtering is. Illustrate your answer with diagrams and...
-
Procurement of commodities is a critical activity for a company's survival, particularly in the manufacturing industry. It is essential in the industry because it standardizes the procurement of...
-
TABLE 6A-1 General Depreciation System: 200% or 150% Declining Balance Switching to Straight-Line* Half-Year Convention Recovery Year 3-Year 5-Year 7-Year 10-Year 15-Year 20-Year 1 33.33 20.00 14.29...
-
The Public Service Commission of State X issued a regulation completely banning all advertising that promotes the use of electricity by any electric utility company in State X. The commission issued...
-
Repeat Example 8.17 for the PengRobinson equation with values of the binary interaction parameter, k12 of 0.025, 0.05, and 0.10. Example 8.17 The following data are available for vaporliquid...
-
The Mesa Table Company has projected the following demand for their dining room table line: There are currently 20 workers on the line, each capable of producing 10 tables per week on regular time,...
-
What do college students do with their time? A survey of 3,000 traditional- age students was taken, with the results as follows: a. Construct a bar chart, a pie chart, and a Pareto chart. b. Which...
-
The balance sheets at the end of each of the first two years of operations indicate the following: Kellman Company Year 2 Year 1 Total current assets $607,308 $555,105 Total investments 66,098 44,074...
-
The chief financial officer for Eagles Beach Wear and Gift Shop is planning for the companys cash flows for the next six months. The following table summarizes the expected accounts receivables and...
-
Ethanol has a heat of vaporization of 38.56 kJ/mol and a normal boiling point of 78.4 C. What is the vapor pressure of ethanol at 15 C?
-
Suppose that 1.15 g of rubbing alcohol (C 3 H 8 O) evaporates from a 65.0-g aluminum block. If the aluminum block is initially at 25 C, what is the final temperature of the block after the...
-
Earlier parts of this chapter suggested that the profession has been mediating auditing crises by decoupling accounting from auditing and that accounting has usually been blamed for audit failures....
-
Prepare a statement of cash flows for Wu using the indirect method.
-
A plate of steel with a central through-thickness flaw of length 16 mm is subjected to a stress of 350 MPa normal to the crack plane. If the yield strength of the material is 1400 MPa what is the...
-
More info The company manufactures a variety of engines for use in farm equipment. At the beginning of the current year, Dansville estimated that its overhead for the coming year would be $300,000....
-
2. Find the partial fraction form of 4826s+3 (a) s3 +382 +2s 49 + 15s s - (b) (c) 3 (d) (e) (s - 2)(s + 3)2 2s+3 (s + 2) s238 14 - (s+1)(s2 2s+5) s3s238-3 (s + 3) (s +9) -
-
What accounts are affected, and in which direction, by the following business activity? Use account names from the expanded accounting equation. Purchase supplies for $ 2 0 0 cash. Question 2 5...
-
Write an equation for the reaction of 1-hexyne with sodium amide in liquid ammonia.
-
Which task is performed by a book-keeper? A. Analysing the trading results B. Entering transactions in the ledger C. Preparing year-end financial statements D. Providing information for...
-
Predict the major product(s) of the reaction between l-valine and: (a) MeOH, H + (b) Di-tert-butyl-dicarbonate (c) NaOH, H 2 O (d) HCl
-
When the N terminus of a peptide is acetylated, the peptide derivative that is formed is unreactive toward phenyl isothiocyanate. Explain. Acetylation PEPTIDE PEPTIDE . N. N. Z
-
Glucagon is a peptide hormone produced by the pancreas that, with insulin, regulates blood glucose levels. Glucagon is comprised of 29 amino acid residues. Treatment with trypsin yields four...
-
Change the rates and GDP depending on the scenario Welcome to Econland Your role: You are responsible for managing the economy of Econland, a medium-sized country, for a period of seven years. You...
-
Explain the role of secondary markets in trading securities and describe how exchanges, such as the New York Stock Exchange, help facilitate the process. 150 words or more so I can better understand!
-
Cyber professionals often advise that one of the best ways to deter fraud is to set your emails for automatic out of office replies so that everyone knows you are out of the office and cybercriminals...

Study smarter with the SolutionInn App


