Top fuel dragsters and funny cars burn nitromethane as fuel according to the balanced combustion equation: Calculate
Question:
Top fuel dragsters and funny cars burn nitromethane as fuel according to the balanced combustion equation: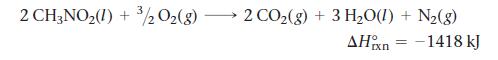
Calculate the standard enthalpy of formation (ΔH°f) for nitromethane.
Transcribed Image Text:
2 CH3NO₂(1) + ³/2O₂(g) 2 CO₂(g) + 3 H₂O(1) + N₂(g) AHxn = -1418 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpy change (H° for the thermal decomposition of silver nitrate according to the following equation is 178.67 kJ: The standard enthalpy of formation of AgNO3(s) is 2123.02...
-
The dry-ash-free proximate composition of coal is 38.86 wt.% volatiles and 61.14 wt.% fixed carbon. Assume the volatiles can be simplified as the following molecule The average molar mass of this...
-
The so-called hydrogen economy is based on hydrogen produced from water using solar energy. The gas is then burned as a fuel: A primary advantage of hydrogen as a fuel is that it is nonpolluting. A...
-
The information to prepare the statement of cash flows comes from all of the following sources except: a. comparative balance sheets. b. additional transaction data about cash provided or used during...
-
Adams, Loomis, and Vogt, three law students who have joined together to open a law practice, are struggling to manage their cash flow. They havent yet built up sufficient clientele and revenues to...
-
Centex Sound Systems purchased merchandise inventory costing $8,000 from Flower Co. on account. Where should Centex record this transaction, and what account is credited? a. Cash payments journal;...
-
Why does Collegiate Apparel need an annual budget? Specifically, what purposes would the budgeting process serve
-
Georgia Cabinets manufactures kitchen cabinets that are sold to local dealers throughout the Southeast. Because of a large backlog of orders for oak and cherry cabinets, the company decided to...
-
On January 1, 2018, Bonita Industries issued its 11% bonds in the face amount of $8090000, which mature on January 1, 2028. The bonds were issued for $9520000 to yield 9%, resulting in bond premium...
-
Golden Wedding Dress Company designs custom wedding dresses for brides to be. The person preparing the adjusting entries at year-end was unable to complete the adjustments due to illness. You have...
-
During photosynthesis, plants use energy from sunlight to form glucose (C 6 H 12 O 6 ) and oxygen from carbon dioxide and water. Write a balanced equation for photosynthesis and calculate H rxn .
-
Use standard enthalpies of formation to calculate H rxn for each reaction. 2 HO(l) +2 SO(8) SO3(8) a. 2 HS(g) + 3 O(g) b. SO2(g) + /2O2(8) c. C(s) + HO(g) CO(g) + H(g) d. NO4(g) + 4 H(g) N(g) +...
-
To test the strength of a 25 20- in. suitcase, forces are applied as shown. If P = 18lb, (a) Determine the resultant of the applied forces, (b) Locate the two points where the line of action of the...
-
Solve X+1U6x-13x+2-4x+5
-
Summarize the selected poster's design format, such as the color, layout, font style, size, space, and the subject's analysis format. Also, analyze how the study started. Such as background and...
-
Income statement Prior year Current year Revenues 782.6 900.0 Cost of sales Selling costs Depreciation (27.0) (31.3) Operating profit 90.4 85.7 Interest Earnings before taxes 85.4 78.2 Taxes (31.1)...
-
View the video at the slide title "Lab: Social Media Post" at time 28:20. Link:...
-
Write a program ranges.py in three parts. (Test after each added part.) This problem is not a graphics program. It is just a regular text program to illustrate your understanding of ranges and loops....
-
The mean time to assemble a product as found from a sample of size 40 is 10.4 minutes. The standard deviation of the assembly times is known to be 1.2 minutes. (a) Find a two-sided 90% confidence...
-
Graph the following conic sections, labeling vertices, foci, directrices, and asymptotes (if they exist). Give the eccentricity of the curve. Use a graphing utility to check your work. 10 5 + 2 cos 0
-
What is the advantage of a 2-D NMR experiment over a 1-D NMR experiment?
-
Why do magnetic field in-homogeneities of only a few parts per million pose difficulties in NMR experiments?
-
Why does NMR lead to a higher contrast in the medical imaging of soft tissues than X-ray techniques?
-
Centurion Co. had the following accounts and balances at December 31: Account Cash Accounts Receivable Prepaid Insurance Supplies Accounts Payable T. Happy, Capital Service Revenue Salaries Expense...
-
Gretchen invests 6200 dollars in a mutual fund on January 1. On March 1, she learns that her fund balance is 3800 dollars, and she then withdraws 1500 dollars. On August 1, her fund balance is 7800...
-
Change the rates and GDP depending on the scenario Welcome to Econland Your role: You are responsible for managing the economy of Econland, a medium-sized country, for a period of seven years. You...

Study smarter with the SolutionInn App


