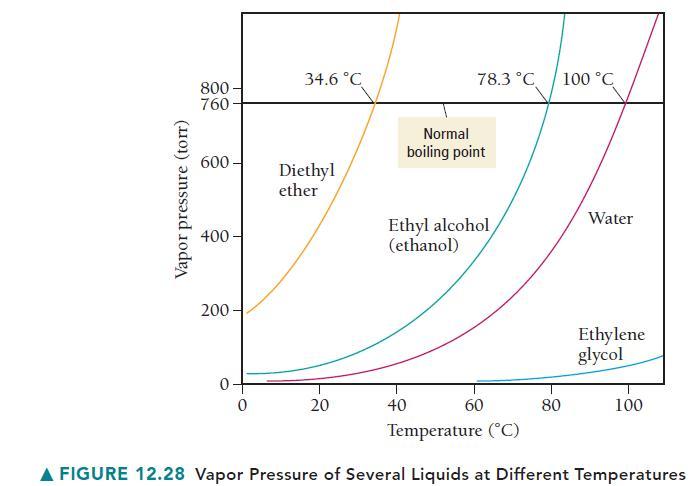
Use Figure 12.28 to estimate the boiling point of water at an external pressure of 200 torr.
Question:
Use Figure 12.28 to estimate the boiling point of water at an external pressure of 200 torr.
(a) 66 °C
(b) 84 °C
(c) 100 °C
(d) 0 °C
Transcribed Image Text:
Vapor pressure (torr) 800- 760 600- 400- 200 0- 0 34.6 °C Diethyl ether 20 78.3 °C 40 Normal boiling point Ethyl alcohol (ethanol) 60 80 100 °C Water Ethylene glycol 100 Temperature (°C) A FIGURE 12.28 Vapor Pressure of Several Liquids at Different Temperatures
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a According to Figure 1228 water has a vapor pressure of 200 torr at about 6...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each question that uses an interval or ratio scale, include a frequency distribution chart, central tendencies (mean, median, and mode), and dispersion metrics (range, standard deviation ...
-
Determine the dimensions of Nul A, Col A, and Row A for the matrices. A = 0 0 4 7 0 0 0
-
Use Figure 11.7 to estimate the boiling point of carbon tetrachloride, CCl 4, under an external pressure of 250 mmHg. 800 760 700 Chloroform- 600 500 400 Diethyl ether 300 200 Water 100 Carbon...
-
As mentioned in the case, the joint venture between Renault and Mahindra & Mahindra (India) dissolved/failed. Speculate what are the possible socio-cultural issues that triggered this dissolution.
-
Refer to the data for Herald Company in Exercise 15-7. Required: Use the direct method to convert the companys income statement to a cash basis.
-
Aimes Corporation's balance sheet at December 31, 2024, is presented below. During 2025, the following transactions occurred. Aimes uses a perpetual inventory system. 1. Aimes paid \(\$ 2,500\)...
-
How do you plan to connect yourself to your new organization? Who are your key audiences, and what messages would you like to convey to them? What are the best modes of engagement? AppendixLO1
-
Joeys Bike Shop sells new and used bicycle parts. Although a majority of its sales are cash sales, it makes a significant amount of credit sales. During 2016, its first year of operations, Joeys Bike...
-
can you show work in a paper 12. Southern Goods is analyzing a proposed project using standard sensitivity analysis. The company expects to sell 4500 units E11 percent. The expected variable cost per...
-
A long copper cylinder 0.6 m in diameter and initially at a uniform temperature of 38?C is placed in a water bath at 93?C. Assuming that the heat transfer coefficient between the copper and the water...
-
Describe the relationship between the state of a substance, its temperature, and the strength of its intermolecular forces.
-
Determine the amount of heat (in kJ) required to vaporize 1.55 kg of water at its boiling point. For water, Hvap = 40.7 kJ/mol (at 100 C). a) 3.50 * 10 3 kJ b) 1.14 * 10 6 kJ c) 2.11 kJ d) 686 kJ
-
Debbie, who is not dependent upon any person, was enrolled as a full-time student at the University of Toronto for eight months during 2016. Debbie provides you with the following information for...
-
Concord Timber Company owns 9,000 acres of timberland purchased in 2014 at a cost of $1.470 per acre. At the time of purchase. the land without the timber was valued at $420 per acre. In 2015,...
-
Foofy computes z-scores for a set of normally distributed exam scores. She obtains a z-score of -3.96 for 8 out of 20 of the students. What do you conclude?
-
Part 1 Recording Using the financial statements for the hypothetical company - Big Box Retailer-record the transactions for the year to the financial statement. The financial statements may be found...
-
Finding Standard Deviation from a Frequency Distribution. In Exercises 37-40, refer to the frequency distribution in the given exercise and compute the standard deviation by using the formula below,...
-
STAR Co. provides paper to smaller companies whose volumes are not large enough to warrant dealing directly with the paper mill. STAR receives 100-feet-wide paper rolls from the mill and cuts the...
-
Write the names and structures of all possible products for the bromination of methane.
-
In Exercises, find the equation of the tangent line at the given point on each curve. 2y 2 - x = 4; (16, 2)
-
A bar having a length of 5 in. and cross-sectional area of 0.7 in. 2 is subjected to an axial force of 8000 lb. If the bar stretches 0.002 in., determine the modulus of elasticity of the material....
-
The rigid pipe is supported by a pin at A and an A-36 steel guy wire BD. If the wire has a diameter of 0.25 in., determine how much it stretches when a load of P = 600 lb acts on the pipe. 4 ft 3 ft...
-
The rigid pipe is supported by a pin at A and an A-36 guy wire BD. If the wire has a diameter of 0.25 in., determine the load P if the end C is displaced 0.075 in. downward. 4 ft 3 ft 3 ft
-
In which transaction cycle would information for retiring long-term debt be most likely to pass between internal and external accounting information systems. Select one: A. the financing cycle B. the...
-
What is the purpose of tests of controls? With reference to the three (3) main risk components of the Audit Risk Model, explain the circumstances where it is NOT appropriate for the Auditor to test...
-
50 If at the end of each month you save 300 for 40 years and earn 8 % annually, what is the Future Value of your savings

Study smarter with the SolutionInn App


