Which is the correct Lewis structure for CO 3 2 - ? b) : |:0=C=0: : ::
Question:
Which is the correct Lewis structure for CO32 -?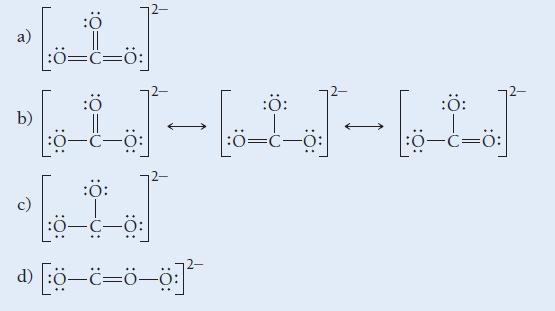
Transcribed Image Text:
ه ده b) :Ö |:0=C=0: :Ö :Ö: - - - :Ö= ھا- ا :Ö: :0–c—0: 20 -=- :Ö: :ة=-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
b ...View the full answer

Answered By

S Mwaura
A quality-driven writer with special technical skills and vast experience in various disciplines. A plagiarism-free paper and impeccable quality content are what I deliver. Timely delivery and originality are guaranteed. Kindly allow me to do any work for you and I guarantee you an A-worthy paper.
4.80+
27+ Reviews
73+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which is the correct Lewis structure for nitrogen trifluoride? a) :F=N-F: F: c) :F-N-F: :F: b) :F-N-F: F: d) :F-N-F-F:
-
Which is the correct Lewis structure for magnesium bromide? a) 2 Mg+ [Br] c) Mg+2 [Br] b) Mg: Br: d) :Mg: Br:
-
A second Lewis structure can be drawn for one of the nucleophiles in Problem 36. (a) Identify it and draw its alternate structure (which is simply a second resonance form), (b) Is there a second...
-
What is the output of the following program: #include using namespace std; void Push(int x[], int y[], int n) { int i = 0, j = n - 1; while (i
-
Kinkel Corporation makes a product with the following standard costs for direct material and direct labor: Direct material: 1.50 meters at $5.40 per meter . . . . . . . $8.10 Direct labor: 0.25 hours...
-
A Bill Smithson runs a second-hand furniture business from a shop which he rents. He does not keep complete accounting records, but is able to provide you with the following information about his...
-
Which limitations cause you to discard the alternative as a possibility?
-
The controller of Shoe Mart Inc. asks you to prepare a monthly cash budget for the next three months. You are presented with the following budget information: The company expects to sell about 20% of...
-
I know headquarters wants us to add that new product line, said Dell Havasi, manager of Billings Companys Office Products Division. But I want to see the numbers before I make any move. Our divisions...
-
The adjusted trial balance at April 30, 2020, for Zhang Co. follows. Debit 101 Cash $ 3,900 106 Accounts receivable 8,800 153 Trucks 27,500 154 Accumulated depreciation, trucks 193 Franchise 13,500...
-
The reaction between hydrogen and oxygen to form water is highly exothermic. Which statement is true of the energies of the bonds that break and form during the reaction? (a) The energy needed to...
-
Draw the Lewis structure (including resonance structures) for nitromethane (CH 3 NO 2 ). For each resonance structure, assign formal charges to all atoms that have formal charge.
-
A man 6 feet tall walks at a rate of 5 feet per second toward a streetlight that is 30 feet high (see figure). The man's 3-foot-tall child follows at the same speed, but 10 feet behind the man. At...
-
Write a Program to Remove the Vowels from a String
-
For each of the metals listed in the table, compute the Pilling-Bedworth ratio. Also, on the basis of this value, specify whether or not you would expect the oxide scale that forms on the surface to...
-
Make an argument that Williams had a right to delay the closing until after August 1.
-
The tank of the air compressor is subjected to an internal pressure of 90 psi. If the inner diameter of the tank is 22 in., and the wall thickness is 0.25 in., determine the stress components acting...
-
The thin-walled cylinder can be supported in one of two ways as shown. Determine the state of stress in the wall of the cylinder for both cases if the piston P causes the internal pressure to be 65...
-
A pressurized spherical tank is made of 0.5-in.-thick steel. If it is subjected to an internal pressure of p = 200 psi, determine its outer radius if the maximum normal stress is not to exceed 15 ksi.
-
Karen wants to have $27,471 in her investment account in 8 years. If her bank offers an annual compound interest rate of 2.6% with monthly compounding, how much should she deposit today?
-
In this task your supervisor has asked you to demonstrate and prove your understanding and ability to use the appropriate charts and tables to present and communicate findings of different categories...
-
Jazz Corporation owns 10% of the Williams Corp. stock. Williams distributed a $11,900 dividend to Jazz Corporation. Jazz Corp.'s taxable income (loss) before the dividend was ($7,100). What is the...

Study smarter with the SolutionInn App


