Which is the correct Lewis structure for nitrogen trifluoride? a) :F=N-F: F: c) :F-N-F: :F: b) :F-N-F:
Question:
Which is the correct Lewis structure for nitrogen trifluoride?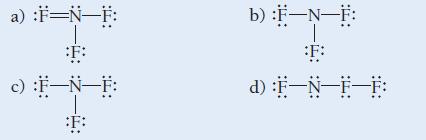
Transcribed Image Text:
a) :F=N-F: F: c) :F-N-F: :F: b) :F-N-F: F: d) :F-N-F-F:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
c...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a Lewis structure for nitrogen pentoxide (N2O5) in which each N is bonded to three O atoms.
-
Which is the correct Lewis structure for CO 3 2 - ? b) : |:0=C=0: : :: - - - := - :: :0c0: 20 -=- :: :=-
-
Which is the correct Lewis structure for magnesium bromide? a) 2 Mg+ [Br] c) Mg+2 [Br] b) Mg: Br: d) :Mg: Br:
-
help me with questions 10, 11 and 12 to answer them for practiceolin data structures Given the following linked list definitions: struct listRec! string name; listRec *link, ;: nodeType *begin; Code...
-
Harmon Household Products, Inc., manufactures a number of consumer items for general household use. One of these products, a chopping board, requires an expensive hardwood. During a recent month, the...
-
Air from a fermentor contains 5% of CO2, which we want to remove. Suggest various removal strategies and identify which one could be economical.
-
How would you complete the brainstorming process if you were Dutton? Has she cataloged all options? Has she made any errors in the process so far?
-
Judge Drago has decided to set up an educational fund for his favorite granddaughter, Emma, who will start college in one year. The judge plans to deposit an amount in a savings account that pays 9...
-
Question 2 With the help of technology, computers can aid Adepa Ghana Limited find valuable information without being programmed about where to look for specific piece of information. This is because...
-
Consider the following relation: CAR_SALE(Car#, Date_sold, Salesperson#, Commission%, Discount_amt) Assume that a car may be sold by multiple salespeople, and hence {Car#, Salesperson#} is the...
-
Which molecule could have an expanded octet? (a) H 2 CO 3 (b) H 3 PO 4 (c) HNO 2
-
Assign formal charges to each atom in the resonance structures of the cyanate ion (OCN ). Which resonance structure is likely to contribute most to the correct structure of (OCN )? A B C |C=N]...
-
Weekly demand for DVD- Rs at a retailer is normally distributed with a mean of 1,000 boxes and a standard deviation of 150. Currently, the store places paper orders faxed to the supplier. Assume 50...
-
What Is Chemical Energy? Definition and Examples
-
The fundamental concern of computer science is determining what can and cannot be automated. The earliest foundations of what would become computer science predate the invention of the modern digital...
-
History of the United States
-
United States History Pearl Harbor attack
-
Briefly explain why, for a small anode-to-cathode area ratio, the corrosion rate will be higher than for a large ratio. Solution
-
What are technical skills At what level are they most important and why?
-
The pipe is subjected to a shear force of V = 8 kip. Determine the shear flow in the pipe at points A and B. 0.2 in. -6 in.
-
The stiffened beam is constructed from plates having a thickness of 0.25 in. If it is subjected to a shear of V = 8 kip, determine the shear-flow distribution in segments AB and CD. What is the...
-
The beam supports a vertical shear of V = 7 kip. Determine the resultant force in segment AB of the beam. 0.5 in. 10 in. -0.5 in. IA 0.5 in. 5 in.
-
Question 9 The following information is available for Astrid Ltd and Duncast Ltd. Astrid 15 000 Duncast 15 000 Units produced and sold Rm Rm Revenues 112.5 112.5 55.0 15.0 Variable costs Fixed costs...
-
What is the quoted price of a bond maturing in 12 years with a coupon rate of 9 percent, paid semiannually, that has a YTM of 13 percent? (Please round to the nearest hundredth)
-
The Deltona Instrument Company has 7 percent coupon bonds on the market with 3 years left to maturity. The bonds make semi-annual payments. If the bond currently sells for $984.60, what is its ytm?

Study smarter with the SolutionInn App


