Which molecule exhibits optical isomerism? H (a) H-C-cl Br (b) Br H H | | C-C-H cl
Question:
Which molecule exhibits optical isomerism?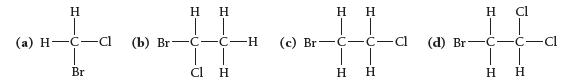
Transcribed Image Text:
H (a) H-C-cl Br (b) Br H H | | C-C-H cl H H H | | (c) Br C-c-c J-H CIH H Η (d) Br H 1 C-H ♫. ICIH C-C-Cl H Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
b This structure is the only one th...View the full answer

Answered By

Sandip Nandnawar
I am a B.E (Information technology) from GECA and also have an M.C.M from The University of RTMNU, MH.
I worked as a software developer (Programmer and TL). Also working as an expert for the last 6 years and deal with complex assessment and projects. I have a team and lead a team of experts and conducted primary and secondary research. I am a senior software engg and senior expert and deal with all types of CSE and IT and other IT-related assessments and projects and homework.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Obtain a Lewis formula of H2N2. This molecule exhibits isomerism (it has two isomers). Sketch the electron-dot formulas of these isomers. Describe the bonding in terms of valence bond theory, and...
-
Out of the following, the alkene that exhibits optical isomerism is (a) 2-methyl-2-pentene (b) 3-methyl-2-pentene (c) 4-methyl-1-pentene (d) 3-methyl-1-pentene
-
Draw the formula of an unsaturated bromide, C5H9Br, that can show a. Neither cis-trans isomerism nor optical activity b. Cis-trans isomerism but no optical activity c. No cis-trans isomerism but...
-
Use the Ratio Test to determine the values of x 0 for which each series converges. 00 X 2k 2 k=1 k
-
Tony Garcia opened a small dryer repair shop, Garcia Repair Shop, on January 2, 2010. The shop also sells a limited number of dryer parts. In January 2011, Garcia realized he had never filed any tax...
-
What are the three major objectives of career development from the organizations viewpoint?
-
Is it the case that global sourcing is really about cost-cutting? LO.1
-
Palmgren Company produces consumer products. The sales budget for four months of the year is presented below. Company policy requires that ending inventories for each month be 25 percent of next...
-
Brian Whippoorwill decided to open a full-service barber shop (Clean Shaven) attached to his residence on property he inherited from his uncle. The business began operations on July 1, year 1, and is...
-
Name this alkane: CH-CH-CH-CH-CH-CH-CH-CH3 CH I CH3 CH3 CH3
-
Which property of carbon is related to its ability to form a large number of compounds? a) Its tendency to form four covalent bonds b) Its ability to form double and triple bonds c) Its tendency to...
-
What are some alternative techniques to traditional interviews?
-
A company which manufactures microwaves advertises that 90% of their microwaves are flawless, requiring no adjustments. Their quality control department tests this percentage on a regular basis. On...
-
A new retail store is being planned for a site that contains 40 ft of soft clay (c 0.075 ft2/day, y = 100 pcf). The clay layer is overlain by 15 ft of sand (y = 112 pcf) and is underlain by dense...
-
Perez Bags (PB) is a designer of high-quality backpacks and purses. Each design is made in small batches. Each spring, PB comes out with new designs for the backpack and for the purse. The company...
-
Find a recent (within the last 12 months) article or economic blog related to price fixing, provide an executive summary of the information. Include an APA reference and/or link. How does the fact...
-
A rectangular block of a material with a modulus of rigidity G=90 ksi is bonded to two rigid horizontal plates. The lower plate is fixed, while the upper plate is subjected to a horizontal force P....
-
Assume Robertson, Inc., paid $289,000 to acquire all the common stock of Dinette Corporation and Dinette owes Robertson $197,000 on a note payable. Immediately after the purchase on September 30,...
-
A. Select a recent issue (paper or online) of Report on Business Magazine, Canadian Business Magazine (online only), Bloomberg Businessweek, Fast Company, The Economist, or another business magazine....
-
An irregular conductor containing an irregular, empty cavity carries a net charge Q. (a) Show that the electric field inside the cavity must be zero. (b) If you put a point charge inside the cavity,...
-
You measure the electric field strength at points directly above the center of a square plate carrying charge spread uniformly over its surface. The data are tabulated in the next column, with x the...
-
A point charge -q is at the center of a spherical shell carrying charge +2q. That shell, in turn, is concentric with a larger shell carrying -3/2 q. Draw a cross section of this structure, and sketch...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


