Which of the protons shown in red is more acidic? H-C-0-H (a) H H-C-0-H H (b)
Question:
Which of the protons shown in red is more acidic?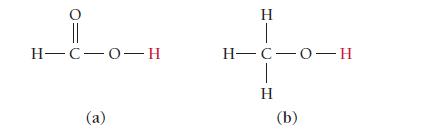
Transcribed Image Text:
H-C-0-H (a) H H-C-0-H H (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a Since the carbon atom in a is bonded to another ...View the full answer

Answered By

Simon kingori
I am a tier-one market researcher and content developer who has been in this field for the last six years. I’ve run the freelancing gamut; from market research, data mining and SEO/SMM to copywriting, Content Development, you name it, I’ve done it. I’m extremely motivated, organized and disciplined – you have to be to work from home. My experience in Freelancing is invaluable- but what makes me a cut above the rest is my passion to deliver quality results to all my clients- it’s important to note, I've never had a dissatisfied client. Backed by a Masters degree in Computer Science from MOI university, I have the required skill set and burning passion and desire to deliver the best results for my clients. This is the reason why I am a cut above the rest. Having taken a Bsc. in computer science and statistics, I deal with all round fields in the IT category. It is a field i enjoy working in as it is dynamic and new things present themselves every day for research and exploration.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Butane (C 4 H 10 ) exhibits only two different kinds of protons, shown here in red and blue. (a) Explain why all four protons shown in red are chemically equivalent. (b) Explain why all six protons...
-
For each of the following compounds, determine whether the two protons shown in red are homotopic, enantiotopic, or diastereotopic: (a) (b) (c) (d) (e) OMe . CI H,
-
Craig's Bowling Inc. operates several bowling centres (for games and equipment sales). The following transactions occurred in November 2017. For each of the following transactions, complete the...
-
Don Masters and two of his colleagues are considering opening a law office in a large metropolitan area that would make inexpensive legal services available to those who could not otherwise afford...
-
Kids Sports Consulting Pty Ltd is a company set up by sports and recreation management students to gain experience in running their own business. It had the following contribution margin income...
-
A large proportion of SMEs fail in this critical time. What do you think are some of the reasons this occurs?
-
Refer to the transactions of CD City in P63A. In P63A, At the beginning of July, CD City has a balance in inventory of $3,400. The following transactions occur during the month of July. July 3...
-
The Colin Division of Crane Company sells its product for $30.00 per unit. Variable costs per unit include: manufacturing. $13.80, and selling and administrative $4.00. Fixed costs are: $322000...
-
Write an equation for the autoionization of water and an expression for the ion product constant for water (K w ). What is the value of K w at 25 C?
-
Define the acid ionization constant and explain its significance.
-
a. Calculate the value of Ka for the following acids: i. 0.0200 mol dm 3 2-aminobenzoic acid, which has a pH of 4.30 ii. 0.0500 mol dm 3 propanoic acid, which has a pH of 3.10 iii. 0.100 mol dm 3...
-
Question 1: You overheard your investment advisor saying, "Don't put all the eggs into the same basket. Explain the meaning of this statement. Explain three (3) reasons of why your investment advisor...
-
* * Audit Procedures for Auditor's Responsibility for Risk Assessment * * In auditing, risk assessment is a critical phase where auditors identify and evaluate risks that may impact the financial...
-
Case # 4 Joseph Joseph, a 19-year-old African American college freshman. Yesterday he spent the afternoon drinking beer and taking shots of vodka with his fraternity brothers. After 6 glasses of beer...
-
1. Kaldor facts [50 points] Kaldor (1961) documented a set of stylized facts on the growth process of industrialized countries. We discussed these facts in lecture 2. Explain if and how the...
-
County has the Investment Activities recorded in its general fund: Tesla Stock: Cost $100, Fair Value on Jan 1x1: $200; Fair Value on Dec 31x2: $300 DJT Stock: Cost: $100; Fair Value on Jan 1x1:...
-
Using the same data as in Exercise 14.2, a researcher tests for a stochastic trend in In(IPt) using the following regression: where the standard errors shown in parentheses are computed using the...
-
Quadrilateral EFGH is a kite. Find mG. E H <105 G 50 F
-
Consider the circular space station in Figure 5.13. Suppose the station has a radius of 15 m and is designed to have an acceleration due to artificial gravity of g/2. Find the speed of the rim of the...
-
A child of mass m - 50 kg sits at the end of a rope of length L = 3.2 m. The other end of the rope is fastened to a ceiling in a gymnasium, and the child travels so that he moves in a horizontal...
-
A car of mass 1700 kg is traveling without slipping on a flat, curved road with a radius of curvature of 35 m. If the cars speed is 12 m/s, what is the frictional force between the road and the tires?
-
ABC company paid 3.00$ on dividends per share over the past year. Assuming dividends will grow at a rate of 15% for the next two years and a constant rate of 5% thereafter. compute the value of ABC...
-
Reference: ACNT 1311- Quickbooks Online 2021 update /(MyBusinesCcourse-Math Revealed) Background information: Martin Smith, a college student and good friend of yours, has always wanted to be an...
-
2) Describe in 2-3 paragraphs the key differences (strengths and weaknesses) of Quantitative versus Qualitative research methods as you interview Millennial and Gen Z participants on the impact of...
Genetic Analysis Genes Genomes And Networks In Eukaryotes 2nd Edition - ISBN: 0199651817 - Free Book

Study smarter with the SolutionInn App


