Would you expect an alloy of iron and vanadium to be substitutional or interstitial? Explain your choice.
Question:
Would you expect an alloy of iron and vanadium to be substitutional or interstitial? Explain your choice. Create a phase diagram for the binary alloy of iron and vanadium based on the tabulated data provided.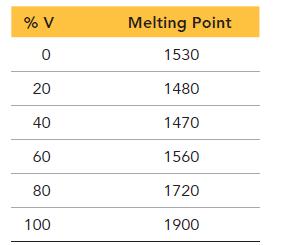
Transcribed Image Text:
% V 0 20 40 60 80 100 Melting Point 1530 1480 1470 1560 1720 1900
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Based on the melting point data provided in the image I would expect an alloy of iron and vanadium t...View the full answer

Answered By

HABIBULLAH HABIBULLAH
I have been tutor on chegg for approx 5 months and had solved a lot of questions.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An alloy of iron and carbon was treated with sulfuric acid, in which only iron reacts. 2Fe(s) + 3H2SO4(aq) Fe2(SO4)3(aq) + 3H2(g) If a sample of alloy weighing 2.358 g gave 0.1152 g of hydrogen,...
-
An alloy of iron (54.7%), nickel (45.0%), and manganese (0.3%) has a density of 8.17 g/cm3. How many iron atoms are there in a block of alloy measuring 10.0 cm 20.0 cm 15.0 cm?
-
The binary phase diagram for the silver (Ag) and germanium (Ge) system is shown in Figure 11-34. Figure 11-34 The silver-germanium phase diagram (for Problem 11-35). (a) Schematically draw the phase...
-
You find a certain stock that had returns of 18 percent, 23 percent, 16 percent, and 9 percent for four of the last five years. If the average return of the stock over this period was 10.3 percent,...
-
Johson Corporation issued bonds twice during 2010. The transactions were as follows: 2010 Jan 1Issued $1,000,000 of 7.5 percent, 10-year bonds dated January 1, 2010, with interest payable on June 30...
-
Suppose that the joint p.d.f. of two random variables X and Y is proportional, as a function of (x, y), to exp[ax2 + by2 + cxy + ex + gy + h], where a > 0, b > 0, and c, e, g, and h are all...
-
Why are each of the BRIC countries viewed as potential candidates for global expansion?
-
Four factors are thought to possibly influence the taste of a soft-drink beverage: type of sweetener (A), ratio of syrup to water (B), carbonation level (C), and temperature (D). Each factor can be...
-
You have gained huge success in your professional career. You would set up an endowment to sponsor scholarships for students from low income families. You expect that scholarships can support $50,000...
-
Create a table comparing and contrasting pyrometallurgy, hydrometallurgy, and electrometallurgy. Include a definition, an application or two, and any relevant notes for each type of metallurgy. Once...
-
Assign each element in the first row of the transition metals, scandium through zinc, to a group member. Have each group member look up on the Internet or in a reputable chemistry reference book the...
-
Two students in a microbiology class are arguing about the origins of biotechnology. One student argued that biotechnology started with the advent of genetic engineering. The other student disagreed,...
-
2. Getting ready for Logarithms and Calculus! a. Fill in the chart and graph the function (I advise practicing on your scientific calculator and desmos. X f(x) = Inx 0 0.5 1 e 10...
-
JoJo Co. had the following balances and information for October. Beg. finished goods inventory = $30 Beg. work in process inventory = $5 Beg. raw materials inventory = $15 End. finished goods...
-
Subway sales have been declining since 2014. In the US, Subway has closed a number of stores due to over-expansion, outdated operations, and uninspiring menus. In Canada, Subway took a different...
-
Harvey Auto Parts purchased a new crane on September 1 for $35,000, paying $10,000 cash and signing a 7%, 12-month note for the remaining balance, interest to be paid at maturity. The crane is...
-
e4(k+1) Find the sum of the series. k = 1 8
-
Refer to the financial statements of American Eagle Outfitters in Appendix B, Urban Outfitters in Appendix C, and the Industry Ratio Report in Appendix D at the end of this book. Required: 1. By what...
-
The figure shows a bolted lap joint that uses SAE grade 8 bolts. The members are made of cold-drawn AISI 1040 steel. Find the safe tensile shear load F that can be applied to this connection if the...
-
Two particles with the same charge are attached to strings that are 80 cm long and that hang from a ceiling as shown in Figure P17.97. If the angle between the strings is ? = 60? and the particles...
-
Consider three particles of charge Q, 2Q, and 4Q, where Q = 2.4 C, arranged as shown in Figure P17.98. If a particle of charge q = -1.5 C is placed at the origin,? (a) What are the components of the...
-
If the electric field is zero in a particular region of space, what does that tell you about the electric potential in that region? Is the potential zero, constant, or something else? Explain.
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


