Write a chemical formula for each molecular model. a. b. C.
Question:
Write a chemical formula for each molecular model.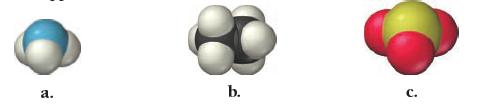
Transcribed Image Text:
a. b. C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a NH ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a policy statement as the HR director stating whether or not office romantic relationships are allowed. If so, under what circumstances? What theoretical ethical perspective did you use to...
-
Write a chemical formula for each compound or ion, and indicate the oxidation state of the group 5A element in each formula: (a) Phosphate ion (b) Arsenous acid (c) Antimony(III) sulfide (d) Calcium...
-
Write a chemical formula for each molecular model. a. b.
-
Can public works increase equilibrium wages?
-
What do we mean when we say companies are offshoring business processes?
-
Starburst is a company focused on solving the pains of data access. It provides a modern solution that addresses data silo and speed of access problems. Starburst product allows customers to analyze...
-
Define insurance from the viewpoint of the individual and of society?
-
Assume a speculator anticipates that the spot rate of the franc in three months will be lower than todays three-month forward rate of the franc, $0.50 = 1 franc. a. How can this speculator use $1...
-
this is a tax law question: please help me. Tom Jonas recently retired as a hockey player with the Toronto Maple Leafs. In the current year, he received his salary of $150,000 from the team and is...
-
White Company has two departments: cutting and finishing. The company uses a job-order costing system and computes a predetermined overhead rate in each department. The cutting department bases its...
-
The presence of one of the ANSWER NOW! following ions within a compound indicates that a compound is soluble with no exceptions. Which ion? (a) OH (b) SO- (c) NO3
-
Calculate the heat of atomization of C 2 H 3 Cl, using the average bond energies in Table 10.3. Data from Review Question 111 The heat of atomization is the heat required to convert a molecule in the...
-
What does it mean for an exception to be bound to an exception handler?
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
Find the complexity of the function given below. void function(int n) { int i, count =0; for(i=1; i*i
-
(a) From the plot of yield strength versus (grain diameter)-1/2 for a 70 Cu-30 Zn cartridge brass, determine values for the constants 0 and ky in Equation 7.7. (b) Now predict the yield strength of...
-
SBS Company have received a contract to supply its product to a Health Care Service Hospital. The sales involve supplying 1,250 units every quarter, the sales price is RM 85 per unit. The Client...
-
A cannon is fired horizontally from a platform (Fig. P2.49). The platform rests on a flat, icy, friction less surface. Just after the shell is fired and while it is moving through the barrel of the...
-
A force is found to be 240 g cm/s 2 . Convert this value into units of newtons.
-
In the U.S. customary system of units, force is measured in units of pounds (abbreviated lb). Suppose the force on an object is 150 lb. Using the conversion factors inside the front cover of this...
-
A fost-growing firm recently paid a dividend of $0.20 per share. The dividend is expected to increase at a 20 percent rate for the next three years. Afterwards, a more stable 11 percent growth rate...
-
What image does it intend to convey? What messages are being communicated to the shareholders? Assess the financial strength of the company and list three audit risk factors you have identified which...
-
Becker Office Service purchased a new computer system on January 1, Year 1, for $38,800. It is expected to have a five-year useful life and a $3,200 salvage value. Becker Office Service expects to...

Study smarter with the SolutionInn App


