Calculate the heat of atomization of C 2 H 3 Cl, using the average bond energies in
Question:
Calculate the heat of atomization of C2H3Cl, using the average bond energies in Table 10.3.
Data from Review Question 111
The heat of atomization is the heat required to convert a molecule in the gas phase into its constituent atoms in the gas phase.
The heat of atomization is used to calculate average bond energies.
Without using any tabulated bond energies, calculate the average C—Cl bond energy from the following data: the heat of atomization of CH4 is 1660 kJ/mol, and the heat of atomization of CH2Cl2 is 1495 kJ/mol.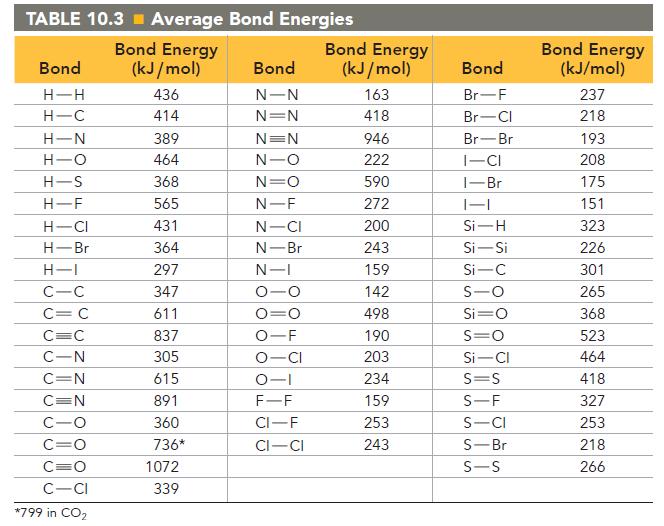
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





