Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals,
Question:
Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 11.6 and 11.7.
a. COCl2 (carbon is the central atom)
b. BrF5
c. XeF2
d. I3–
Examples 11.6
Write a hybridization and bonding scheme for bromine trifluoride, BrF3.
Examples 11.7
Write a hybridization and bonding scheme for acetaldehyde,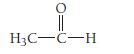
Transcribed Image Text:
O H3C-C-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a sp Csp0p Clp Csp ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 11.6 and 11.7. a. SO...
-
Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping...
-
Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping...
-
Please help Gary earned \( \$ 97,000 \) as an executive. Gary, who is single, supported his half sister, who lives in a nursing home. His half sister had no income during the year. Gary received the...
-
Molle Corp. had total variable costs of $170,000, total fixed costs of $120,000, and total revenues of $250,000. Compute the required sales in dollars to break even.
-
Describe the advantages and disadvantages of the approaches to international market entry discussed in this chapter.
-
The following figures have been extracted from the books of a manufacturing company. All jobs pass through the companys two departments: Working department (Rs) Finishing department (Rs) Materials...
-
Arthur Andersen LLP was the auditor for Enron, WorldCom, Waste Management and other companies that committed fraud. Andersen was forced to shut its doors forever after a U.S. Department of Justice...
-
Bravo Manufacturing Company is negotiating with a customer for the lease of a large machine manufactured by Bravo. The machine has a cash price of $ 9 9 0 , 0 0 0 . Bravo wants to be reimbursed for...
-
Consider the structure of the amino acid alanine. Indicate the hybridization about each interior atom. H H H H
-
Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 11.6 and 11.7. a. CH 2 Br 2 ...
-
Steam is to be condensed on the shell side of a 1-shell-pass and 8-tube-passes condenser, with 50 tubes in each pass, at 30°C (h fg = 2431 kJ/kg). Cooling water (c p = 4180 J/kg·K) enters...
-
As the human resource manager, how would you evaluate the training needs of your staff? How can you ensure that the training you would provide is effective? What data might be used to make your...
-
MARYLAND CORPORATION manufactures three liquid products - Alpha, Beta and Gamma using a joint process with direct materials, direct labor and overhead totaling $560,000 per batch. In addition, the...
-
Three common organizational structures. Mention one organization for each organizational structure which is following a specific organizational structure. Also, provide support to your answer by...
-
You are a retail manager at Kitchen Nightmare, a relatively new store at the mall that sells mostly items for kitchens, like forks, oven mitts, etc.. You have been open since the fall of 2021 and...
-
Examine the extent to which the Department of Veteran Affairs has established any processes or procedures to ensure knowledge retention of departing employees. Why is it important to manage the...
-
Refer to the financial information at the back of the book for Under Armour and Columbia Sportswear. Required Part A. The Ratio Analysis Model For each company, determine: The debt-to-equity ratio Is...
-
The electric field due to a line charge is given by where l is a constant. Show that E is solenoidal. Show that it is also conservative. E =
-
Two long slits 0.10 mm wide, separated by 0.20 mm, in an opaque screen are illuminated by light with a wavelength of 500 nm. If the plane of observation is 2.5 m away, will the pattern correspond to...
-
In a two-slit setup, each slit is 0.020 mm wide. These apertures are illuminated by plane waves of yellow sodium light ( = 589.6 nm). The resulting Fraunhofer fringe pattern consists of 11 narrow...
-
What is the relative irradiance of the subsidiary maxima in a three-slit Fraunhofer diffraction pattern? Draw a graph of the irradiance distribution, when = 2b, for two and then three slits.
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


