Write Lewis structures for each molecule or ion. Use expanded octets as necessary. a. C. CIFS Cl3PO
Question:
Write Lewis structures for each molecule or ion. Use expanded octets as necessary.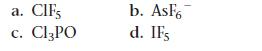
Transcribed Image Text:
a. C. CIFS Cl3PO b. AsF6 d. IFs
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a F F Chlorine pe...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write Lewis structures for each molecule or ion. Use expanded octets as necessary. a. PF c. SF4 b. 13 d. Gel
-
Write Lewis structures for each molecule or ion. Include resonance structures if necessary and assign formal charges to all atoms. If necessary, expand the octet on the central atom to lower formal...
-
Write Lewis structures for the following species, including all resonance forms, and show formal charges: (a) HCO2- , (b) CH2NO2- . Relative positions of the atoms are as follows: H C
-
Discuss sources of revenues Explain the importance of prices and volume in revenue budgeting Clarify why revenues are often ignored in nursing budgets Discuss when revenues should be considered in...
-
The payroll department at the Bonnie P Manufacturing Company has defined the following record structure for employee records. All fields are required. The employee's Social Security number serves as...
-
Should capital budgeting decisions be made solely on the basis of a projects NPV, with no regard to the other criteria? Explain your answer.
-
Customer profitability analysis. Zoots Suits is a ready-to-wear suit manufacturer with headquarters in Toronto. Zoots has three customers: April Department Stores, a large department store chain that...
-
In divisional income statements prepared for Wilborne Construction Company, the Pay-roll Department costs are charged back to user divisions on the basis of the number of payroll checks, and the...
-
please answer with explanation Legal and Professional accounting. 16 services considered (2 ) Selling expenses Administrative expenses None of the above Other income and expenses O Financing costs o
-
Your firm has decided to try two approaches to estimating a valuation allowance for sales returns. Required: a. First, your firm decides to estimate that 10% of all sales will eventually be returned....
-
Which compound shown here has the stronger nitrogennitrogen bond? The shorter nitrogennitrogen bond? H,NNH,, HNNH
-
Write the Lewis structure for each molecule (octet rule not followed). a. BBr 3 b. NO c. ClO 2
-
Implement the following method that returns the maximum element in an array: public static > E max(E[] list) Write a test program that prompts the user to enter 10 integers, invokes this method to...
-
In the regression model \(y=\beta_{1}+\beta_{2} x+e\), assume \(x\) is endogenous and that \(z\) is a valid instrument. In Section 10.3.5, we saw that \(\beta_{2}=\operatorname{cov}(z, y) /...
-
Using data on the Maltese economy, Apap and Gravino \({ }^{19}\) estimate a number of versions of Okun's Law. Their quarterly data run from 1999Q1 to 2012Q4 and can be found in the data file apap....
-
Consider a stationary model that combines the \(\operatorname{AR}(2)\) model \(y_{t}=\delta+\theta_{1} y_{t-1}+\theta_{2} y_{t-2}+e_{t}\) with an \(\mathrm{AR}(1)\) error model \(e_{t}=ho...
-
Consider the AR(1) model \(y_{t}=\delta+\theta y_{t-1}+e_{t}\) where \(|\theta|)=0\) and \(\operatorname{var}\left(e_{t} \mid I_{t-1} ight)=\sigma^{2}\). Let \(\bar{y}_{-1}=\sum_{t=2}^{T} y_{t}...
-
Prove (9.31) . = BE (GS;S; G) BT, B = (B), B-T (9.31)
-
The following true stresses produce the corresponding true plastic strains for a brass alloy: What true stress is necessary to produce a true plastic strain of 0.25? True Stress (psi) 50,000 60,000...
-
The baseball player A hits the ball from a height of 3.36 ft with an initial velocity of 34.8 ft/s. 0.14 seconds after the ball is hit, player B who is standing 15 ft away from home plate begins to...
-
When you dive to a depth of 12.50 ft in seawater, what is the pressure?
-
A water storage tank is on the roof of a factory building and the surface of the water is 50.0 ft above the floor of the factory. If a pipe connects the storage tank to the floor level and the pipe...
-
An open tank contains ethylene glycol at 25C. Compute the pressure at a depth of 3.0 m.
-
Which investment should I choose? Bond A: BBB Corporate bond, Price=$1,100, Par=$1,000, Coupon rate=4% (semiannual coupons), 13 years to maturity Bond B: BBB Corporate bond, Price=$5,900, Par=$5,000,...
-
During October, total equivalent units of output were 86,000 using the weighted-average method. The information about the beginning and ending inventories for October were as follows: Units in...
-
Q12. PDQ has an expected sales volume of $1,000,000 with a variable cost ratio of 55%, and fixed costs of $200,000. What sales volume would be necessary to achieve a $100,000 after-tax profit when...

Study smarter with the SolutionInn App


