Given the following electrostatic potential diagrams, comment on the expected solubility of CH 4 in water and
Question:
Given the following electrostatic potential diagrams, comment on the expected solubility of CH4 in water and NH3 in water.
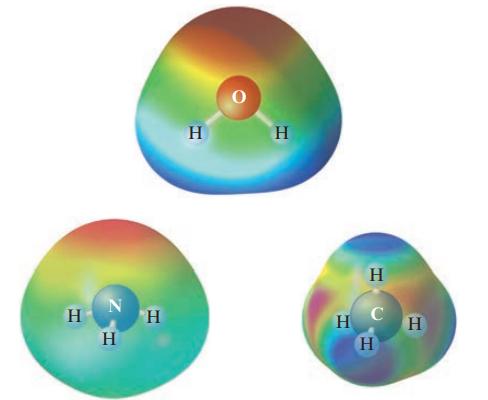
Transcribed Image Text:
н N H Н H Н Н Н с Н Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The electrostatic potential maps of CH4 and NH3 show that CH4 is a nonpolar molecule while NH3 is a ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
The following electrostatic potential diagrams represent H2, HCl, or NaCl. Label each, and explain your choices. (a) (b)
-
The following electrostatic potential diagrams represent CH4, NH3, or H2O. Label each, and explain your choices. a. C. b.
-
Consider the following electrostatic potential diagrams for some covalent compounds. Which of the represented compounds would not be soluble in water? a. b. C. d. e. f.
-
The people on Coral Island buy only juice and cloth. The CPI market basket contains the quantities bought in 2016. The average household spent $60 on juice and $30 on cloth in 2016 when the price of...
-
The comparative balance sheet of Flack Inc. for December 31, 2013 and 2012, is shown as follows: The following additional information was taken from the records: a. The investments were sold for...
-
Please use Python. The Iris data set is:...
-
Q2 What are the responsibilities of the IT department?
-
A job order cost card for Hal's Computer Services appears at the top of the next page. Complete the missing information. The profit factor in the organization's cost-plus contract is 30 percent of...
-
Builtrite stock is currently selling for $30 a share. You purchase 250 shares and later the stock is selling for $39 a share. Without using margin, what is your ROI? Group of answer choices 20% 25%...
-
The price of Cilantro, Inc., stock will be either $60 or $80 at the end of the year. Call options are available with one year to expiration. T-bills currently yield 6 percent. a. Suppose the current...
-
In terms of Raoults law, distinguish between an ideal liquidliquid solution and a nonideal liquidliquid solution. If a solution is ideal, what is true about H soln , T for the solution formation, and...
-
Some ionic compounds contain a mixture of different charged cations. For example, wstite is an oxide that contains both Fe 2+ and Fe 3+ cations and has a formula of Fe 0.950 O 1.00 . Calculate the...
-
Calculation the speed of a proton (mo = 1.67 X 10-27kg) whose kinetic energy is exactly half. (a) Its total energy (b) Its rest energy.
-
Consider a circuit where one or more capacitors is discharged through a light bulb filament with a resistance of 3.0 0.3 . Assume that the resistance of the filament is constant (to within the stated...
-
3. For a vibrating string of length with fixed ends, each mode of vibration can be written as where wk ux(x, t) = M* sin(wxt + k) sin(x) and Mk, Ok are determined by initial conditions. For all k >...
-
The combined weight of the load and the platform is 200 lb, with the center of gravity located at G. If a couple moment of M = 900 lb ft is applied to link AB, determine the angular velocity of links...
-
Due In: 06:48:23 Questions Question 1 (4) O Question 2 (8) Question 2 of 2 A company sold $150,000 bonds and set up a sinking fund that was earning 8.5% compounded semi-annually to retire the bonds...
-
Find the point on the graph of f(x) = x which is closest to the point (6, 27). How close is the closest point?
-
A biologist takes 300 water samples from a lake. He uses an indicator solution to find that 225 of the samples are in the pH range between 5.5 and 6.5. The mean pH is calculated to be 6.0. Estimate...
-
Periwinkle Company is a multinational organization. Its Parts Division is located in Lavender Land, while its Assembly Division is located in North Orchid. During the current year Periwinkle Companys...
-
Propose a mechanism for the following transformation: . 1) Excess MeMgBr 2) H20
-
An ideal gas undergoes an expansion from the initial state described by P i , V i , T to a final state described by P f , V f , T in a. A process at the constant external pressure P f . b. In a...
-
Show the reagents you would use to achieve the following transformation:
-
. In explain the problem Distinguish between a meteor, a meteoroid, a meteorite, an asteroid, and a comet
-
Question 5 of 12 - / 1 View Policies Current Attempt in Progress On January 1, 2020, Larkspur Co. leased a building to Crane Inc. The relevant information related to the lease is as follows. 1. The...
-
detectable before inspection of finished goods. Spoiled units are disposed of at zero net disposal value. Chiplist uses the weighted - average method of process costing. Summary data for September 2...

Study smarter with the SolutionInn App


