Nitrogen gas (N 2 ) and hydrogen gas (H 2 ) react to form ammonia gas (NH
Question:
Nitrogen gas (N2) and hydrogen gas (H2) react to form ammonia gas (NH3).
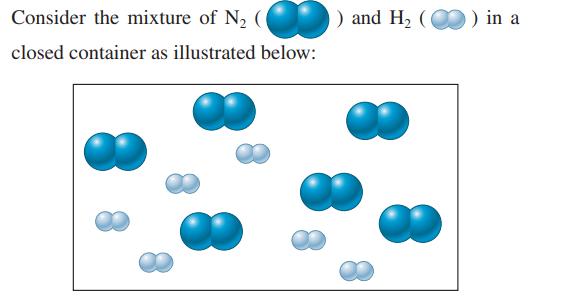
Assuming the reaction goes to completion, draw a representation of the product mixture. Explain how you arrived at this representation.
Transcribed Image Text:
Consider the mixture of N₂ ( closed container as illustrated below: ) and H₂ () in a
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The balanced chemical equation for the reaction of nitrogen gas N2 and hydrogen g...View the full answer

Answered By

Abigael martinez
I have been a tutor for over 3 years and have had the opportunity to work with students of all ages and backgrounds. I have a strong belief that all students have the ability to learn and succeed if given the right tools and support. I am patient and adaptable, and I take the time to get to know each student's individual learning style in order to best support their needs. I am confident in my ability to help students improve their grades and reach their academic goals.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Nitrogen (N2) and hydrogen (H2) react to form ammonia (NH3). Consider the mixture of N2 and H2 shown in the accompanying diagram. The blue spheres represent N, and the white ones represent H. Draw a...
-
Nitrogen monoxide and oxygen react to form nitrogen dioxide. Consider the mixture of NO and O2 shown in the accompanying diagram. The blue spheres represent N, and the red ones represent O. (a) Draw...
-
Nitrogen and hydrogen gases react to form ammonia gas as follows: N2 (g) + 3 H2 2NH3 (g) At a certain temperature and pressure, 1.2 L of N2 reacts with 3.6 L of H2. If all the N2 and H2 are...
-
Use the DerivaGem software to value a five-year collar that guarantees that the maximum and minimum interest rates on a LIBOR-based loan (with quarterly resets) are 7% and 5% respectively. The LIBOR...
-
Algoe expects to invest $1,000 annually for 40 years to yield an accumulated value of $154,762 on the date of the last investment. For this to occur, what rate of interest must Algoe earn? (Use Table...
-
Create a user-defined function to calculate the value of a common stock using the earnings model that was introduced in Chapter 8 on page 246. a. What arguments will you need to accept in order to...
-
LO7 What is an installment sale?
-
Define X as the number of under filled bottles from a filling operation in a carton of 24 bottles. Sixty cartons are inspected and the following observations on X are recorded: Values 0 1 2 3...
-
Please help asap. Please provide answer to all parts. Finance Problem As corporate treasurer, you have to pay $ 2 3 million in one year and again in two years. Bonds of all maturities currently yield...
-
Using data from case exhibit 8 calculate the following:1) 2017 EVA for the North American Dermatology Division2) 2017 EVA bonus payout for a manager assuming that the managers salary is $300,000 and...
-
For the preceding question, which of the following equations best represents the reaction? 4NH3 + 4N a. 6N + 6H b. N + HNH3 c. N + 3H NH3 d. N + 3H 2NH3 e. 2N + 6H 4NH3
-
What mass of silver chloride can be prepared by the reaction of 100.0 mL of 0.20 M silver nitrate with 100.0 mL of 0.15 M calcium chloride? Calculate the concentrations of each ion remaining in...
-
The trial balance of Stone Environmental Services at October 31, 2014, is shown on the next page. The data needed for the month-end adjustments are as follows: a. Unearned consulting revenue that...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Porch Pirates An InsuranceQuotes.com survey showed that 8% of Americans had a holiday package stolen from outside their front door. Consider the random selection of four Americans. Use the...
-
GATE 2024-EE Question
-
GATE 2024-EE Question
-
GATE 2024-EE Question
-
Calculate the cutting time needed to tap a 3/4 2 in. hole using a cutting speed of 30 sfpm.The tap has 10 tpi.
-
Write the general quadratic equation y2 - 8y - 4x + 28 = 0 in standard form. Determine the vertex, focus, and directrix of the parabola defined by this equation. Sketch a graph.
-
Rank these organic compounds in terms of increasing water solubility (from least water soluble to most water soluble). CH3CHCHCOH A CH3 CH3CHCH=C B CH3 || CH3CHCHCCH3 C
-
Draw bond-line structures for all constitutional isomers with molecular formula C 4 H 9 Cl.
-
(S)-2-Iodopentane undergoes racemization in a solution of sodium iodide in DMSO. Explain.
-
A new CEO was hired to revive the floundering Champion Chemical Corporation. The company had endured operating losses for several years, but confidence was emerging that better times were ahead. The...
-
Please complete the trial balance. Bug-Off Exterminators provides pest control services and sells extermination products manufactured by other companies. Following is the company's unadjusted trial...
-
Case 1 (30 point) Continuing with the business idea innovation that you described earlier, you are asked to prepare a budget for the first quarter of 2021 related to the sales budget, expected cash...

Study smarter with the SolutionInn App


