Two variations of the octahedral geometry (see Table 4.1) are illustrated below. Which of the compounds/ions Br
Question:
Two variations of the octahedral geometry (see Table 4.1) are illustrated below.
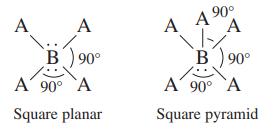
Which of the compounds/ions Br3-, CIF3, XeF4, SF4, PF5, CIF5, and SF6 have these molecular structures?
Transcribed Image Text:
A A B 90° A 90° A Square planar 90° A A ⁹0⁰ A 1/ B) 90° A 90° A Square pyramid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The square planar molecular structure is shown on the left in the imageand the square pyramidal molecular structure is shown on the right The followin...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Write a paper describing a current problem or challenge, how ML or AI could help with this problem, and then describe what this application would be able to do.
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
It is proposed to use water instead of refrigerant-134a as the working fluid in air-conditioning applications where the minimum temperature never falls below the freezing point. Would you support...
-
Are devaluations of pegged exchange rates totally unexpected?
-
Do Prob. 10.8-1. The Tinker Construction Company is ready to begin a project that must be completed in 12 months. This project has four activities (A, B, C, D) with the project network shown next....
-
What would you predict might be the most revealing type of information gathered through the 360-degree feedback?
-
Elm Manufacturing Company (ELM) is a small manufacturer of backpacks located in Rochelle, Illinois. You have access to ELM's electronic records (via the "Sales 2017 - 4th Quarter" dataset provided...
-
When a monopolist switches from charging a single price to practicing perfect prize discrimination, it reduces
-
Indigo is the dye used in coloring blue jeans. The term navy blue is derived from the use of indigo to dye British naval uniforms in the eighteenth century. The structure of the indigo molecule is a....
-
Show how 2s orbitals combine to form bonding and antibonding molecular orbitals. Show how 2p orbitals overlap to form bonding, bonding, antibonding, and antibonding molecular orbitals.
-
Mitch cares only about how much he can write. Because a pen will write 7 miles of text and a pencil will write 35 miles of text, Mitch considers them perfect 5-to-1 substitutes. If the price of pens...
-
Compare the alternatives that Bergerac is considering for its decision. Include: Comparison of make versus buy option in the type of operation that Bergerac is looking to integrate. You do not need...
-
Let A, B, C and D be non-zero digits, such that CD is a two-digit positive integer. BCD is a three-digit positive integer generated by the digits B, C and D. ABCD is a four-digit positive integer...
-
1.) An aluminum tube is clamped with rigid plates using four bolts as shown. The nut on each bolt is tightened one turn from 'snug'. The thickness of the plate may be considered insignificant in this...
-
4.21 Case Study Competency IV.1RM Determine diagnosis and procedure codes and groupings according to official guidelines. Competency IV.1 Validate assignment of diagnostic and procedural codes and...
-
W.E.B Dubois taught the book called "The State" to his students at Atlanta University. Who wrote this book
-
Let X1,..., Xn be a random sample from a n(, 2) population. (a) If is unknown and 2 is known, show that Z = n( - 0)/ is a Wald statistic for testing H0: = 0. (b) If 2 is unknown and is known, find...
-
As of January 1, 2018, Room Designs, Inc. had a balance of $9,900 in Cash, $3,500 in Common Stock, and $6,400 in Retained Earnings. These were the only accounts with balances in the ledger on January...
-
An unknown salt is either NaCN, NaC 2 H 3 O 2 , NaF, NaCl, or NaOCl. When 0.100 mole of the salt is dissolved in 1.00 L of solution, the pH of the solution is 8.07. What is the identity of the salt?
-
A 0.050-M solution of the salt NaB has a pH of 9.00. Calculate the pH of a 0.010-M solution of HB.
-
Arrange the following 0.10 M solutions in order of most acidic to most basic. KOH, KNO3, KCN, NHCH, HC1
-
MULTIPLE CHOICE ASAP PLS WILL UPVOTE 11 Multiple Choice $14,400. $85,600. $9,400. $5,760. $28,000
-
Instructions The net income reported on the income statement for the current year was $371,000. Depreciation recorded on store equipment for the year amounted to $16.880 Balances of the current asset...
-
Which of the following is true? Lower-level managers are responsible for preparing the entire budget. The budget and the administration of the budget are the responsibility of management. The flow of...

Study smarter with the SolutionInn App


