A 0.20 mol sample of magnesium burns in air to form 0.20 mol of solid MgO. What
Question:
A 0.20 mol sample of magnesium burns in air to form 0.20 mol of solid MgO. What amount (moles) of oxygen (O2) is required for a complete reaction?
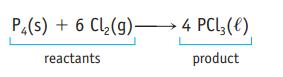
Transcribed Image Text:
P4(s) + 6 Cl₂(g) 4 PCL3 (1) reactants product
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
In the chemical reaction between magnesium Mg and o...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Magnesium burns in air to produce magnesium oxide, MgO, and magnesium nitride, Mg3N2. Magnesium nitride reacts with water to give ammonia. Mg3N2(s) + 6H2O(l ) 3Mg(OH)2(s) + 2NH3(g) What volume of...
-
A 21.496-g sample of magnesium is burned in air to form magnesium oxide and magnesium nitride. When the products are treated with water, 2.813 g of gaseous ammonia are generated. Calculate the...
-
(a) Define the following in terms of gain or loss of hydrogen with one example each : (i) oxidation (ii) reduction (b) When a magnesium ribbon is heated, it burns in air to form magnesium oxide....
-
Question 2A) Explain the differences between right issues and bonusissues. Your answer should include the advantages and disadvantagesof both right issues and bonus issues. [ 8marks}b) Explain pos 0...
-
Suppose that upon Polands entering the European Union, it is discovered that the cost of automobile production in Poland is 20,000 while it is 30,000 in Germany. Suppose that the EU, which has a...
-
The first-order line of 589-nm light falling on a diffraction grating is observed at a 15.5o angle. How far apart are the slits? At what angle will the third order be observed?
-
What are some ways that firms generate ideas for capital projects? AppendixlLO1
-
Since the early 1990s, woodstove sales have declined from 1,200,000 units per year to approximately 100,000 units per year. The decline has occurred because of (1) Stringent new federal EPA...
-
Subject :- Aviation Finance. * PLEASE SHOW IN DETAIL OF THE CALCULATION / WORKING. * PLEASE DON'T REPEAT THE QUESTIONS. JUST DIRECT TO THE ANSWER . FOR EXAMPLE :- Question 3. (Answer) * I'LL UPVOTE...
-
Equal amounts of two acidsHCl and HCO 2 H (formic acid)are placed in aqueous solution. When equilibrium has been achieved, the HCl solution has a much greater electrical conductivity than the HCO 2 H...
-
Oxidation of 1.00 g of carbon monoxide, CO, produces 1.57 g of carbon dioxide, CO 2 . How many grams of oxygen were required in this reaction? P4(s) + 6 Cl(g) 4 PCL3 (1) reactants product
-
Write the structure of the tetrahedral intermediate formed in each of the reactions given in Problem 20.7. Using curved arrows, show how each tetrahedral intermediate dissociates to the appropriate...
-
Suppose that you own the only company in the market to produce a certain product, and therefore you can determine the market price P dollars for each unit. Due to government regulations, the price of...
-
describes how the blast pressure front can bounce off solid, immovable obstacles and be redirected in another direction in a linear angle to the angle of the obstacle hat was struck
-
As the accounting clerk, you are tasked by the CFO to determine the cost of goods sold of Del Mundo Company for the year ended December 31, 2020. During Operating cost data annd inventory account...
-
Using the ideas of kinetic particle theory when you come home from school and open the door you can smell food being cooked
-
The following information relates to Salamat Corporation for the last year.Salamat uses direct labor hours as an overhead base. Estimated direct labor hours 360,000 hours Estimated manufacturing...
-
In alphabetical order below are balance sheet items for Karol Company at December 31, 2017. Prepare a balance sheet following the information given below: Accounts payable...
-
How does Kant answer Humes bundle theory of self? Do you think he is successful?
-
One mole of an ideal gas is subjected to the following changes. Calculate the change in temperature for each case if C V,m = 3/2R. a. q = 425 J, w = 185 J b. q = 315. J, w = 315 J c. q = 0, w = 225 J
-
Consider the adiabatic expansion of 0.500 mol of an ideal monatomic gas with C V ,m = 3/2R. The initial state is described by P = 6.25 bar and T = 300.K. a. Calculate the final temperature if the gas...
-
A pellet of Zn of mass 31.2 g is dropped into a flask containing dilute H 2 SO 4 at a pressure of P = 1.00 bar and temperature of T = 300.K. What is the reaction that occurs? Calculate w for the...
-
The following selected transactions were completed during August between Summit Company and Beartooth Co. Both companies use the net method under a perpetual inventory system. Aug. 1 Summit Company...
-
Martin Technical Institute (MTI), a school owned by Lindsey Martin, provides training to individuals who pay tuition directly to the school. MTI also offers training to groups in off-site locations....
-
Nautical Creations is one of the largest producers of miniature ships in a bottle. An especially complex part of one of the ships requires special production equipment that is not useful for other...

Study smarter with the SolutionInn App


