A solution of KMnO 4 absorbs light at 540 nm (page 206). What is the frequency of
Question:
A solution of KMnO4 absorbs light at 540 nm (page 206). What is the frequency of the light absorbed? What is the energy of one mole of photons with λ = 540 nm?
Data given on Page 206
Transcribed Image Text:
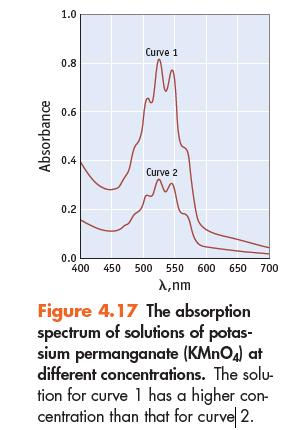
Absorbance 1.0 0.8 0.6 0.4 0.2 Curve 1 Curve 2 0.0 400 450 500 550 600 650 700 X,nm Figure 4.17 The absorption spectrum of solutions of potas- sium permanganate (KMnO4) at different concentrations. The solu- tion for curve 1 has a higher con- centration than that for curve 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To find the frequency of light absorbed and the energy of one mole of photons with a wavelength of 5...View the full answer

Answered By

Gaurav Soni
Teaching was always an area where I can pursue my passion. I used to teach my friends and junior during my school and college life. After completing my professional qualification (chartered accountancy) and before joining my job, I also joined an organization for teaching and guidance to my juniors. I had also written some articles during my internship which later got published. apart from that, I have also given some presentations on certain amendments/complex issues in various forms.
Linkedin profile link:
https://www.linkedin.com/in/gaurav-soni-38067110a
5.00+
7+ Reviews
13+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Apex Company invested $14,400 in new equipment. The more efficient new equipment was expected to reduce operating cash outflows over the next five years by the following: yr1 = $8000 yr2= $6000 yr3=...
-
How much energy is available in visible light? How much energy does sunlight deliver to Earth? How efficient are plants at converting light energy into chemical energy? The answers to these questions...
-
According to the quantum mechanics, electromagnetic radiation of frequency can be regarded as consisting of photons of energy h where h = 6.626 10 -34 J.s is the Plancks constant. (a) What is the...
-
F udy A big part of communication is sharing personal information with another person. Some information we believe we have the right to be our own and should remain private. However, the degree to...
-
Under what circumstances are strikes and lockouts justified in place of mediation or arbitration?
-
The Texas Department of Transportation (TxDOT) is considering two designs for crash barriers along a reconstructed portion of 1-10. Design 2B will cost $3 million to install and $135,000 per year to...
-
Would you want to work for a company like Google? Why or why not?
-
The balance sheet for Lemay Company reports the following information on July 1, 2010. Lemay decides to redeem these bonds at 101 after paying semiannual interest. Prepare the journal entry to record...
-
which it is entitled, within 2 months of its year end or if Space's current debt equity ratio doubles within the 3 years. Invincible Ltd.'s Chief Operations Officer (COO) will coordinate the launch...
-
Suppose hydrogen atoms absorb energy so that electrons are excited to the n = 7 energy level. Electrons then undergo these transitions, among others: (a) n = 7 n = 1; (b) n = 7 n = 6; and (c) n = 2 ...
-
Using spdf and noble gas notations, write electron configurations for atoms of the following elements. (Try to do this by looking at the periodic table but not at Table 7.3.) (a) Strontium, Sr. This...
-
A cube of wood floating in water supports a 200-g mass resting on the center of its top face. When the mass is removed, the cube rises 2.00 cm. Determine the volume of the cube.
-
What role do formalized processes and protocols play in highly structured organizations, and how can organizations balance the need for structure with the imperative for flexibility and innovation ?
-
In what ways do decision-makers balance quantitative data with qualitative insights to optimize complex strategic choices, especially in high-stakes business environments where traditional metrics...
-
Reflect on your semester. How do you plan onmeasuringyour professionalgrowth in the future? What were the most challenging topics to you? What topics felt more intuitive/easy? How do you plan on...
-
Aside from shareholders, who do you believe is the second stakeholder in whose interests the company should be concerned? Justify your response What will you do to ensure the company's success...
-
a) What CSR did your organization do - how did it improve your organization's image? b) If your organization did not do any CSR, as the boss, what CSR activities would you suggest doing and why?
-
Complete the generalization of the Pythagorean theorem begun in Problem 59 of section 0.3 by showing that A+B = C in figure 12 these being the areas of similar regions built on the two legs and the...
-
For a nonzero constant a, find the intercepts of the graph of (x 2 + y 2 ) 2 = a 2 (x 2 - y 2 ). Then test for symmetry with respect to the x-axis, the y-axis, and the origin.
-
The structural assembly supports the loading shown. Draw the moment diagrams for each of the beams. Take I = 100(10 6 ) mm 4 for the beams and A = 200 mm 2 for the tie rod. All members are made of...
-
The contilevered beam is supported at one end by a 1/2 in.-diameter suspender rod AC and fixed at the other end B. Determine the force in the rod due to a uniform loading of 4 k/ft. E = 29(10 3 ) ksi...
-
The beam AB has a moment of inertia I = 475 in 4 and rests on the smooth supports at its ends. A 0.75-in-diameter rod CD is welded to the center of the beam and to the fixed support at D. If the...
-
A new CEO was hired to revive the floundering Champion Chemical Corporation. The company had endured operating losses for several years, but confidence was emerging that better times were ahead. The...
-
Please complete the trial balance. Bug-Off Exterminators provides pest control services and sells extermination products manufactured by other companies. Following is the company's unadjusted trial...
-
Case 1 (30 point) Continuing with the business idea innovation that you described earlier, you are asked to prepare a budget for the first quarter of 2021 related to the sales budget, expected cash...

Study smarter with the SolutionInn App


