Question:
Using spdf and noble gas notations, write electron configurations for atoms of the following elements. (Try to do this by looking at the periodic table but not at Table 7.3.)
(a) Strontium, Sr. This element is named for a town in Scotland.
(b) Zirconium, Zr. The metal is exceptionally resistant to corrosion and so has important industrial applications. Moon rocks show a surprisingly high zirconium content compared with rocks on Earth.
(c) Rhodium, Rh. This metal is used in jewelry and in catalysts in industry.
(d) Tin, Sn. The metal was used in the ancient world. Alloys of tin (solder, bronze, and pewter) are important.
Data Given in Table 7.3
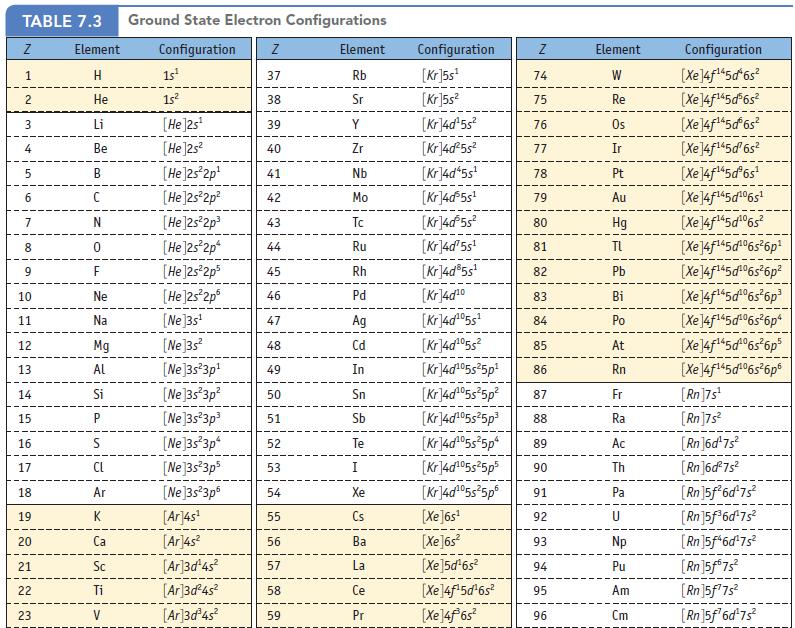
![24 25 26 27 28 29 30 31 32 33 34 35 36 328213588218212 Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr [Ar]3d4s](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/2/3/6/19765390725abe231698236197619.jpg)
Transcribed Image Text:
TABLE 7.3
Z
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
Element
H
He
Li
Be
B
C
N
0
F
Ne
Na
Mg
AL
Si
P
S
CL
Ar
K
Ca
Sc
Ti
Ground State Electron Configurations
Configuration Z
37
38
15¹
15²
[He]2s¹
[He]2s²
[He]2s²2p¹
[He]2s²2p²
[He]2s²2p³
[He]2s²2p*
[He]2s²2p5
[He]2s²2p6
[Ne]3s¹
[Ne]3s²
[Ne]3s²3p¹
[Ne]3s²3p²
[Ne]3s²3p³
[Ne]3s²3p4
[Ne]3s²3p5
[Ne]3s²3p6
[Ar]4s¹
[Ar]4s²
[Ar]3d¹45²
[Ar]3d²4s²
[Ar]3d²4s²
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
Element
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
Cs
2131812
Ba
La
Ce
Pr
Configuration
[kr]5s¹
[kr]5s²
Z
74
75
76
77
78
79
80
81
82
83
[kr]4d¹05s¹
84
[kr]4d¹055²
85
[kr]4d¹05s²5p¹
86
[kr]4d¹05s²5p²
87
[kr]4d¹05s²5p³
88
[kr]4d¹05s²5p4
89
[kr]4d¹05s25p5 90
[kr]4d¹05s²5p6
91
92
93
94
95
96
[kr]4d¹5s²
[kr]4d²5s²
[kr]4d45s¹
[kr]4d³5s¹
[kr]4d 5s²
[kr]4d²5s¹
[kr]4d³5s¹
[kr]4d1⁰
[Xe]6s¹
[Xe]6s²
[Xe]5d¹6s²
[Xe]4f¹5d¹6s²
[Xe]4f 6s²
Element
W
Re
Os
Ir
1212121=121512
Pt
Au
Hg
TL
Pb
At
Rn
1212121212115
Np
Am
Cm
Configuration
[Xe]4f¹5d*6s²
[Xe]4f¹45d³6s²
[Xe]4f¹45dº6s²
[Xe]4f¹45d²6s²
[Xe]4f¹5d²6s¹
[Xe]4f¹45d¹⁰6s¹
[Xe]4f45d6s?
[Xe]4f1%5d106s®6p*
[Xe]4f145d6s®6p
[Xe]4f¹45d¹º6s²6p³
[Xe]4f145d6s®6p*
[Xe]4f¹45d¹º6s²6p³
[Xe]4f145d*6s?6p®
[Rn]7s¹
[Rn]7s²
[Rn]6d¹7s²
[Rn]6d²7s²
[Rn]5f²6d¹7s²
[Rn]5f³6d¹7s²
[Rn]5f*6d¹7s²
[Rn]5fº7s²
[Rn]5f¹7s²
[Rn]5f¹6d¹7s²

![24 25 26 27 28 29 30 31 32 33 34 35 36 328213588218212 Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr [Ar]3d4s](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/2/3/6/19765390725abe231698236197619.jpg)






