Ammonia gas can be prepared by the reaction of a metal oxide such as calcium oxide with
Question:
Ammonia gas can be prepared by the reaction of a metal oxide such as calcium oxide with ammonium chloride.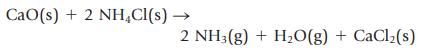
If 112 g of CaO and 224 g of NH4Cl are mixed, what is the limiting reactant, and what mass of NH3 can be produced?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





