Boron forms a series of compounds with hydrogen, all with the general formula B x H y
Question:
Boron forms a series of compounds with hydrogen, all with the general formula BxHy.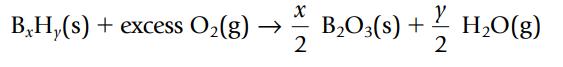
If 0.148 g of one of these compounds gives 0.422 g of B2O3 when burned in excess O2, what is its empirical formula?
Transcribed Image Text:
X B Hy(s) + excess O₂(g) → 2 Y B₂O3(s) + s) + 2/1/201 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
To determine the empirical formula of the compound you need to find the ratio of the moles of boron ...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Silicon and hydrogen form a series of compounds with the general formula Si x H y . To find the formula of one of them, a 6.22-g sample of the compound is burned in oxygen. All of the Si is converted...
-
Fluorine is so reactive that it forms compounds with materials inert to other treatments. (a) When 0.327 g of platinum is heated in fluorine, 0.519 g of dark red, volatile solid forms. What is its...
-
What makes the water move in a drip coffee brewer? The electrical pump pushes the water past the check valve and the heater up to the sprayhead The heater creates bubbles of steam, which expand and...
-
Given the following marginal utility schedule for good X and good Y for an individual A, given that the price of X and the price of Y are both $10, and that the individual spends all his income of...
-
Explain the importance of respect for people in JIT.
-
A beverage can with a diameter of 65 mm and a height of 120 mm has a uniform temperature of 5C when it is removed from the refrigerator. The can is set on a table in a room with an ambient air...
-
A simple random sample of size 100 is selected from a population with p .40. a. What is the expected value of ? b. What is the standard error of ? c. Show the sampling distribution of . d. What does...
-
BONDS ISSUED AT A PREMIUM Wang Corporation issued the following bonds at a premium: Date of issue and sale: .....March 1, 20-1 Principal amount: .......$250,000 Sale price of bonds: .......103...
-
Act AOL Mail Saved undational 6 The Foundational 15 (Static) (LO2-1, LO2-2, LO2-3, LO2-4) [The following information applies to the questions displayed betow] Sweeten Company had no jobs in progress...
-
Saccharin, an artificial sweetener, has the formula C 7 H 5 NO 3 S. Suppose you have a sample of a saccharin-containing sweetener with a mass of 0.2140 g. After decomposition to free the sulfur and...
-
Iodine is made by the following reaction (a) Name the two reactants. (b) If you wish to prepare 1.00 kg of I 2 , what masses of NaIO 3 and NaHSO 3 are required? (c) What is the theoretical yield of I...
-
To analyse the relationship between strategic management and SHRM.
-
7. A psychiatrist is testing a new ADHD Medication, which seems to have the potentially harmful side effect of increasing the heart rate. For a sample of 50 clinical study participants whose pulse...
-
Determine the type of engagement that your colleague completed for the client. Justify the selected engagement type for the client. Assess the purpose of each financial statement for the client's...
-
Mills Corporation acquired as a long-term investment $235 million of 8% bonds, dated July 1, on July 1, 2024. Company management has classified the bonds as an available-for-sale investment. The...
-
A force of 28 pounds acts on the pipe wrench shown in the figure below. 18 in. 30 (a) Find the magnitude of the moment about O by evaluating ||OA x F||. (0 0 180) Use a graphing utility to graph the...
-
Module 1 1. There has been a rise in cases of measles in RI. The RI Health Department is wondering if the rate of MMR vaccinations has declined since the start of the COVID-19 pandemic. The...
-
Lou Ann Staas opened a software consulting firm that immediately paid $9,000 for a computer. Was Staas's payment an expense of the business? Explain your answer.
-
Assume Eq. 6-14 gives the drag force on a pilot plus ejection seat just after they are ejected from a plane traveling horizontally at 1300 km/h. Assume also that the mass of the seat is equal to the...
-
Identify the starting materials needed to make each of the following acetals: (a) (b) (c) OEt
-
Using ethanol as your only source of carbon atoms, design a synthesis for the following compound:
-
Propose an efficient synthesis for each of the following transformations: (a) (b)
-
The following has been summarised after a review for Memories Limited the bank statement, dated 31st July 2020, and the general ledger (ignore GST): | In ledger but not on bank statement Outstanding...
-
#of units Cost per unit Cost of Goods Available for Sale $ 5,000 # of units sold Cost per unit Cost of Goods Sold # of units in ending Cost per unit inventory 100 $ 50.00 Ending Inventory 100 $50.00...
-
Sievert Co. sold merchandise to Vargas Co. on account, $157,600, terms FOB shipping point. 2/10, 1/30. The cost of the merchandise sold is $94.550 Sievert Co. paid freght of $2.100. Assume that all...

Study smarter with the SolutionInn App


