Calculate the molar mass of each hydrated compound. The water of hydration is included in the molar
Question:
Calculate the molar mass of each hydrated compound. The water of hydration is included in the molar mass.
Given Data
 (a). Ni(NO3)2 ∙ 6 H2O
(a). Ni(NO3)2 ∙ 6 H2O
(b). CuSO4 ∙ 5 H2O
Transcribed Image Text:
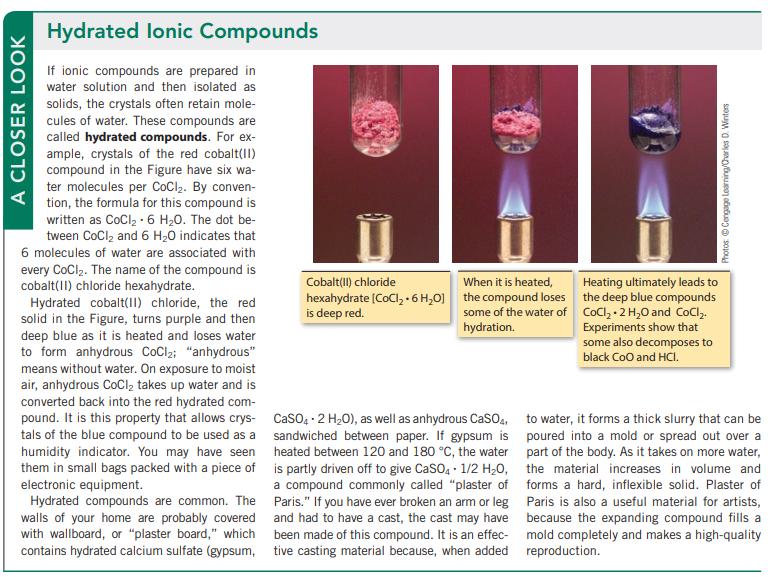
A CLOSER LOOK Hydrated lonic Compounds If ionic compounds are prepared in water solution and then isolated as solids, the crystals often retain mole- cules of water. These compounds are called hydrated compounds. For ex- ample, crystals of the red cobalt(II) compound in the Figure have six wa- ter molecules per CoCl₂. By conven- tion, the formula for this compound is written as CoCl₂-6 H₂0. The dot be- tween CoCl₂ and 6 H₂O indicates that 6 molecules of water are associated with every CoCl₂. The name of the compound is cobalt(II) chloride hexahydrate. Hydrated cobalt(II) chloride, the red solid in the Figure, turns purple and then deep blue as it is heated and loses water to form anhydrous CoCl₂; "anhydrous" means without water. On exposure to moist air, anhydrous CoCl₂ takes up water and is converted back into the red hydrated com- pound. It is this property that allows crys- tals of the blue compound to be used as a humidity indicator. You may have seen them in small bags packed with a piece of electronic equipment. Hydrated compounds are common. The walls of your home are probably covered with wallboard, or "plaster board," which contains hydrated calcium sulfate (gypsum, Cobalt(II) chloride When it is heated, hexahydrate (CoCl₂ + 6H₂O] the compound loses is deep red. some of the water of hydration. CaSO4 2 H₂O), as well as anhydrous CaSO4, sandwiched between paper. If gypsum is heated between 120 and 180 °C, the water is partly driven off to give CaSO4 1/2 H₂O, a compound commonly called "plaster of Paris." If you have ever broken an arm or leg and had to have a cast, the cast may have been made of this compound. It is an effec- tive casting material because, when added Photos Cengage Learning/Charles D. Winters Heating ultimately leads to the deep blue compounds CoCl₂.2 H₂O and CoCl₂. Experiments show that some also decomposes to black CoO and HCI. to water, it forms a thick slurry that can be poured into a mold or spread out over a part of the body. As it takes on more water, the material increases in volume and forms a hard, inflexible solid. Plaster of Paris is also a useful material for artists, because the expanding compound fills a mold completely and makes a high-quality reproduction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To calculate the molar mass of a hydrated compound we need to add the molar mass of each element in ...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Calculate the molar mass of each hydrated compound. Note that the water of hydration is included in the molar mass. Given Data (a). H 2 C 2 O 4 2 H 2 O (b). MgSO 4 7 H 2 O, Epsom salt A CLOSER LOOK...
-
Elaborate figures given below Price per pair (dollars) 105 90 75 60 45 30 15 0 10 20 30 I E Equilibrium IS 40 50 60 70 Quantity of tennis shoes (thousands of pairs per year) 80 90 100 D
-
d^w Consider a beam that can be described by Euler-Bernoulli Beam ODE EI q(x). Assume the beam 10 meters long, has an area moment of inertial of 8e-5 m, has one fixed-end boundary condition (w' = 0...
-
When a company's bookkeeper started to prepare the monthly bank reconciliation, the cash account showed a balance of P528.600. At the end of the month, the following information was available from...
-
Your supervisor, Jesse Baker, has asked you to begin working on data design tasks for the new information system, which will be implemented as a relational database. You will need to identify the...
-
Figure 4.6a and Figure 4.6b in your textbook each show an acceleration vector. Which of the following statements are correct?\ Select all that apply.\ The acceleration vector in the figure labeled...
-
1 What may the artifacts suggest about the deeper beliefs and values, or underlying assumptions?
-
On December 1, delivery equipment was purchased for $7,200. The delivery equipment has an estimated useful life of four years (48 months) and no salvage value . Using the straight-line depreciation...
-
On January 1, 2021, HGC Camera Store adopted the dollar-value LIFO retail inventory method. Inventory transactions at both cost and retail, and cost indexes for 2021 and 2022 are as follows:...
-
Analysis of a 10.0-g sample of apatite (a major component of tooth enamel) showed that it was made up of 3.99 g Ca, 1.85 g P, 4.14 g O, and 0.020 g H. List these elements based on relative amounts...
-
You are given 0.10-g samples of K, Mo, Cr, and Al. List the samples in order of the amount (moles), from smallest to largest.
-
Valentine Industries manufactures three models of a product in a single plant with two departments: Cutting and Assembly. The company has estimated costs for each of the three product models: the...
-
Discussion Questions a. How might the development of personal health records change the role of HIM professionals who work for healthcare organizations? b. What types of personal health record...
-
One of your jobs as a Business Development Officer is to make sure that your company's brand, product, and package are available (e.g. 1kilo pack Vida hotdog, half kilo Vida hotdog, etc.). One of...
-
A major corporation has hundreds of major development projects underway at the same time. Each project is meant to go through an independent formal technical review process, but all of the scheduling...
-
1. What are the external factors (i.e., technology, society, infrastructure) present that have led to the potential for incorporation of this new form of transportation? 2. What analysis process must...
-
executive summary about the three most significant critical success factors for effective health and safety management systems
-
On April 1, Year 1, Company P purchased 85% of S Company for total consideration of $357,000, which included $30,000 of contingent consideration as measured according to GAAP at fair value. Each...
-
Use the method of Example 4.29 to compute the indicated power of the matrix. 1 0 1
-
Draw the product(s) obtained from hydroboration-oxidation of (E)-3-methyl-3-hexene.
-
The products obtained from hydroboration-oxidation of cis-2-butene are identical to the products obtained from hydroboration-oxidation of trans-2-butene. Draw the products and explain why the...
-
As the pressure is increased at 45C, ice I is converted to ice II. Which of these phases has the lower density?
-
How does the community or people provide services from nonprofits (what challenges does the community or people face in seeking help from nonprofits)
-
Stockholders Equity Transactions, Journal Entries, and T-Accounts The stockholders equity of Fremantle Corporation at January 1 follows: 8 Percent preferred stock, $110 par value, 20,000 shares...
-
can i know the calculations and citations?

Study smarter with the SolutionInn App


