Calculate the molar mass of each hydrated compound. Note that the water of hydration is included in
Question:
Calculate the molar mass of each hydrated compound. Note that the water of hydration is included in the molar mass.
Given Data
(a). H2C2O4 ∙ 2 H2O
(b). MgSO4 ∙ 7 H2O, Epsom salt
Transcribed Image Text:
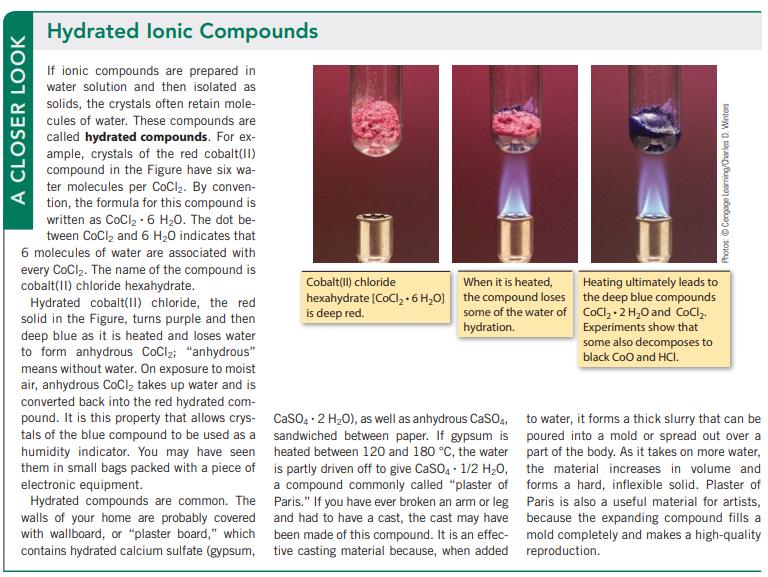
A CLOSER LOOK Hydrated lonic Compounds If ionic compounds are prepared in water solution and then isolated as solids, the crystals often retain mole- cules of water. These compounds are called hydrated compounds. For ex- ample, crystals of the red cobalt(II) compound in the Figure have six wa- ter molecules per CoCl₂. By conven- tion, the formula for this compound is written as CoCl₂-6 H₂0. The dot be- tween CoCl₂ and 6 H₂O indicates that 6 molecules of water are associated with every CoCl₂. The name of the compound is cobalt(II) chloride hexahydrate. Hydrated cobalt(II) chloride, the red solid in the Figure, turns purple and then deep blue as it is heated and loses water to form anhydrous CoCl₂; "anhydrous" means without water. On exposure to moist air, anhydrous CoCl₂ takes up water and is converted back into the red hydrated com- pound. It is this property that allows crys- tals of the blue compound to be used as a humidity indicator. You may have seen them in small bags packed with a piece of electronic equipment. Hydrated compounds are common. The walls of your home are probably covered with wallboard, or "plaster board," which contains hydrated calcium sulfate (gypsum, Cobalt(II) chloride When it is heated, hexahydrate (CoCl₂ + 6H₂O] the compound loses is deep red. some of the water of hydration. CaSO4 2 H₂O), as well as anhydrous CaSO4, sandwiched between paper. If gypsum is heated between 120 and 180 °C, the water is partly driven off to give CaSO4 1/2 H₂O, a compound commonly called "plaster of Paris." If you have ever broken an arm or leg and had to have a cast, the cast may have been made of this compound. It is an effec- tive casting material because, when added Photos Cengage Learning/Charles D. Winters Heating ultimately leads to the deep blue compounds CoCl₂.2 H₂O and CoCl₂. Experiments show that some also decomposes to black CoO and HCI. to water, it forms a thick slurry that can be poured into a mold or spread out over a part of the body. As it takes on more water, the material increases in volume and forms a hard, inflexible solid. Plaster of Paris is also a useful material for artists, because the expanding compound fills a mold completely and makes a high-quality reproduction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a H2C2O4 2 H2O The molar mass of H2C2O4 2 H2O is molar mass o...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Calculate the molar mass of each hydrated compound. The water of hydration is included in the molar mass. Given Data (a). Ni(NO 3 ) 2 6 H 2 O (b). CuSO 4 5 H 2 O A CLOSER LOOK Hydrated lonic...
-
d^w Consider a beam that can be described by Euler-Bernoulli Beam ODE EI q(x). Assume the beam 10 meters long, has an area moment of inertial of 8e-5 m, has one fixed-end boundary condition (w' = 0...
-
A nonvolatile organic compound Z was used to make up two solutions. Solution A contains 5.00 g of Z dissolved in 100 g of water, and solution B contains 2.31 g of Z dissolved in 100 g of benzene. A...
-
ABC Ltd prepares its financial statements to 31 October each year. Its trial balance at 31 October 2019 was as follows: Premises-cost Manufacturing plant-cost Office equipment-cost Accumulated...
-
Define the term system architecture. Define the term scalability, and explain why it is important to consider scalability in system design.
-
Johnny Cake Ltd. has 8 million shares of stock outstanding selling at $22 per share and an issue of $40 million in 10 percent annual coupon bonds with a maturity of 17 years, selling at 94.0 percent...
-
1 Gather evidence (preferably by asking people) about how the culture affects behaviour, and whether they think it helps or hinders performance.
-
Bolton Securities is about to implement a drug-testing procedure for company employees. In a recent anonymous survey, 20% of Boltons employees admitted to using illegal drugs. The random drug testing...
-
Accounting, 9e Help System Announcements Question 2 CALCULATOR Len Jason started his own consulting firm, Jason Consulting, on June 1, 2022. The trial balance at June 30 is as follows PRINTER VERSION...
-
Analysis of a 10.0-g sample of apatite (a major component of tooth enamel) showed that it was made up of 3.99 g Ca, 1.85 g P, 4.14 g O, and 0.020 g H. List these elements based on relative amounts...
-
You are given 0.10-g samples of K, Mo, Cr, and Al. List the samples in order of the amount (moles), from smallest to largest.
-
Samantha deposits $1,500 into the Park Street Bank. The account pays 1.12% annual interest, compounded daily. To the nearest cent, how much is in the account at the end of three nonleap years?
-
There are two electric motors that can provide 100 hp. Alphamotor can be purchased at $1,250 and has an efficiency of 74%, anestimated life of 10 years, and estimated maintenance costs of$50/year....
-
Locate a quote about leadership and post it. As you consider the quote, explain what is appealing about this quote and/or the person who is quoted. Does the quote validate or contradict a principle...
-
Express in terms of logarithms of x, y, z, or w. (a) log(xz) (b) loge (c) log6z
-
Question 1 (10 marks) What should influence your sourcing decisions? Question 2 (10 marks) Why would an organization consider outsourcing? Question 3 (20 marks) Consider Figure 2 (Source: Taylor,...
-
discuss the factors that are driving changes in the South African airline industry.
-
On April 1, Year 1, Company P purchased 85% of S Company for total consideration of $357,000, which included $30,000 of contingent consideration as measured according to GAAP at fair value. Each...
-
Consider model (9.18). What is the effect on the model parameter estimates, their standard errors, and the goodness-of-fit statistics when (a) The times at risk are doubled, but the numbers of deaths...
-
Use (U/V) T = (T - kP)k to calculate (U/V) T for an ideal gas.
-
Under what conditions are H and U for a reaction involving gases and/or liquids or solids identical?
-
Predict the product(s) for each of the following transformations: a. b. c. d. e. f. 1) , THF 2) ,, NaOH 1) BH3 THF 2) H202, NaOH
-
According to IFRS, the pension obligation should be based on Select one: a. the remaining years of serviceboth vested and non-vestedusing future salary levels. b. all years of serviceboth vested and...
-
Yuri Co. operates a chain of gift shops. The company maintains a defined contribution pension plan for its employees. The plan requires quarterly installments to be paid to the funding agent, Whims...
-
Sales value and physical value alfocation

Study smarter with the SolutionInn App


