Calculate the pH of a 0.12 M aqueous solution of the base aniline, C 6 H 5
Question:
Calculate the pH of a 0.12 M aqueous solution of the base aniline, C6H5NH2 (Kb = 4.0 × 10−10).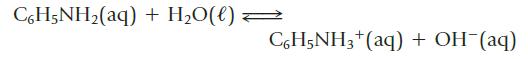
Transcribed Image Text:
CoH5NHz(aq) +H,O(l)< C6H5NH3+ (aq) + OH-(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
To calculate the pH of a 012 M aqueous solution of the base aniline C6H5NH2 you can use the concept ...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Lets take a look at the extensive career of Helen Frankenthaler (1928-2011), who found a way to incorporate all of the innovative techniques artists were experimenting with starting in 1945. Her...
-
Calculate the pH of a 0.15 M aqueous solution of aluminum chloride, AlCl3. The acid ionization of hydrated aluminum ion is and Ka is 1.4 10-5.
-
Calculate the pH of a 0.15 M aqueous solution of zinc chloride, ZnCl2. The acid ionization of hydrated zinc ion is and Ka is 2.5 10-10.
-
Sketch the six graphs of the x- and y-components of position, velocity, and acceleration versus time for projectile motion with x 0 = y 0 = 0 and 0 < 0 < 90.
-
What are the advantages and disadvantages of having an employer draw attention to situations where employees use some of their work time to do non-job-related activities? Does labeling this employee...
-
1. The project that Nationwide undertook was quite clearly a success. What made this possible? Discuss three different practices that helped Nation wide pull this off. Use examples from the case...
-
Identify and explain the four coverage sections of the PAP
-
Sherwin- Williams is a national paint manufacturer and retailer. The company is segmented into five divisions: Paint Stores (branded retail location), Consumer (paint sold through stores such as...
-
Calculate profit in Excel and show formulae: Make the summary of Scenarios with the case of a Mexican company that all its sales are made in Europe, but its raw material is purchased in the United...
-
Suppose you have $5,000 in savings when the price level index is at 100. (a) If inflation pushes the price level up by 10 percent, what will be the real value of your savings? (b) What is the real...
-
Hemoglobin (Hb) can form a complex with both O 2 and CO. For the reaction at body temperature, K is about 200. If the ratio [HbCO]/[HbO 2 ] comes close to 1, death is probable. What partial pressure...
-
Give an example of a law defining a crime that, although held to be constitutional by the U.S. Supreme Court, has been declared unconstitutional by some state courts. Why can such a result occur in...
-
What is the formula for calculating return on investment (ROI)?
-
Air enters a 5.5-cm-diameter adiabatic duct with inlet conditions of \(\mathrm{Ma}_{1}=2.2, T_{1}=250 \mathrm{~K}\), and \(P_{1}=60 \mathrm{kPa}\), and exits at a Mach number of...
-
At the various activity levels shown, Taylor Company incurred the following costs. Required: Identify each of these costs as fixed, variable, or mixed. Units sold 20 40 60 80 100 a. Total salary cost...
-
Indicate whether each of the following types of transactions will either (a) increase stockholders' equity or (b) decrease stockholders' equity: 1. expenses 2. revenues 3. stockholders' investments...
-
The following selected transactions were completed by Lindbergh Delivery Service during October: 1. Received cash from issuing capital stock, \($75,000\). 2. Paid rent for October, \($4,200\). 3....
-
Follow the instruction in problem 45 for f(x) = (sin2 x - 3 tan x) / cos x. Let f(x) = (x3 + 3x - 5) / (x2 + 4). a. Evaluate f(1.38) and f(4.12). (b) Construct a table of values for this function...
-
DEPARTMENT DATA EMPLOYEE DATA EmployeeNumber FirstName Mary Rosalie Richard George Alan 3 4 5 7 8 9 855555ES 12 13 14 15 16 17 Create the database tables in SQL or ACCESS: 18 19 20 PROJECT DATA Ken...
-
Determine the support reactions at and . Take E = 200 MPa, I = 300(10 6 ) mm 4 , A = 21(10 3 ) mm 2 for each member. - 5 m 300 kN m, 3 9. (2, 4 m 2
-
Determine the structure stiffness matrix K for the frame. Take E = GPa, I = 350(10 6 ) mm 4 , A = 15(10 3 ) mm 2 for each member. Joints at and are pins. 2 60 kN 3 |1 2) 2 m - - 2 m 2 4 m
-
Determine the support reactions at pins and . Take E = 200 GPa, I = 350(10 6 ) mm 4 , A = 15(10 3 ) mm 2 for each member. 2 60 kN 3 - 2 m 2 m 4 m 3) 9
-
ILLU ung, 1/ Intermediate Accounting 17e (ACC 334-335- Assignment Gradebook ORION Downloadable Textbook ent NEXT CALCULATOR FULL SCREEN PRINTER VERSION RACK Question 4 On May 1, 2020, Sarasota Inc....
-
X M HUNTER MONTEVERDE, U X Sircon Platform clearninglab.litmos.com/assessment/question?courseld=10889424&moduleld=16564537&questionId=7220591&LPId=204201 unter. X odf Z Insurance Agent &... care...
-
Company ABC manufactures radios and uses direct labor hours (DLH) as its allocation base. The following information relates to the companys manufacturing overhead data for 2021: Budgeted output...

Study smarter with the SolutionInn App


