Consider only transitions involving the n = 1 through n = 4 energy levels for the hydrogen
Question:
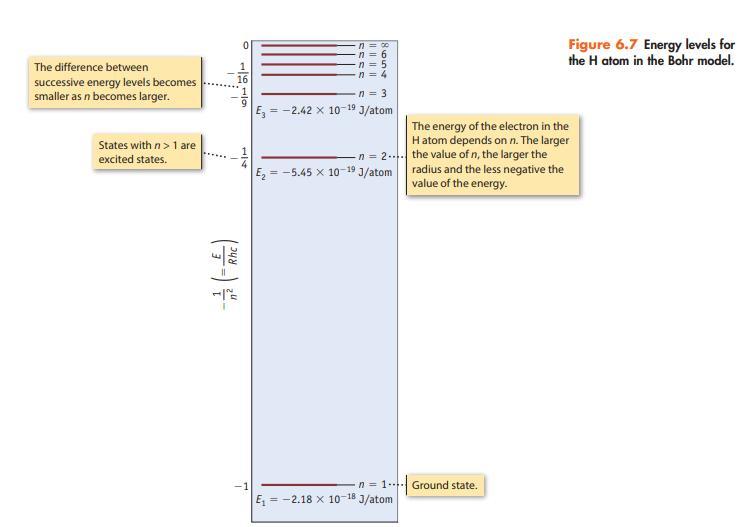
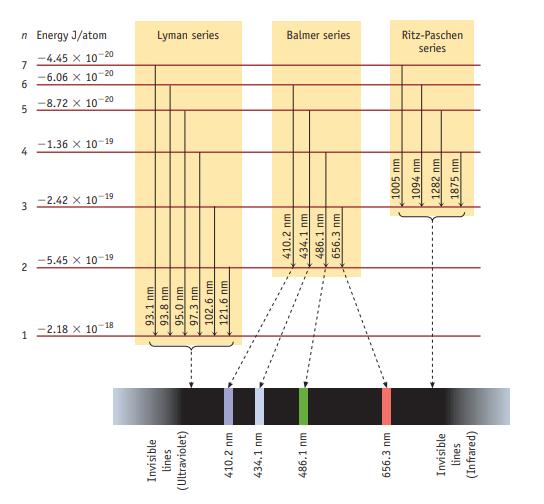
Consider only transitions involving the n = 1 through n = 4 energy levels for the hydrogen atom (see Figures 6.7 and 6.10).
(a) How many emission lines are possible, considering only the four quantum levels?
(b) Photons of the lowest energy are emitted in a transition from the level with n =__________ to a level with n =__________ .
(c) The emission line having the shortest wavelength corresponds to a transition from the level with n =__________ to the level with n =__________ .
Data given in figure 6.7
Data given in figure 6.10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





