Consider only transitions involving the n = 1 through n = 5 energy levels for the H
Question:
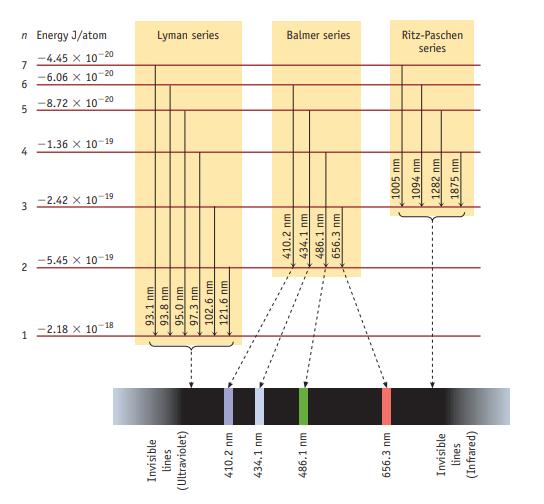
Consider only transitions involving the n = 1 through n = 5 energy levels for the H atom (see Figures 6.7 and 6.10).
(a) How many emission lines are possible, considering only the five quantum levels?
(b) Photons of the highest frequency are emitted in a transition from the level with n= __________ to a level with n =__________ .
(c) The emission line having the longest wavelength corresponds to a transition from the level with n =__________ to the level with n = __________.
Data given in figure 6.7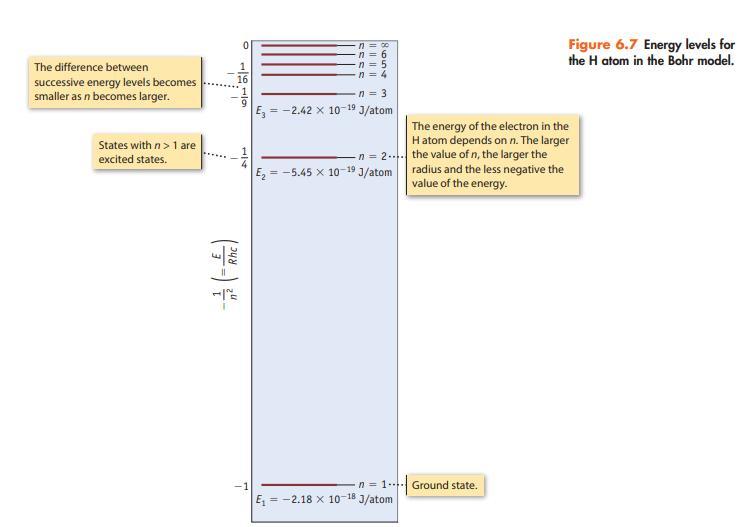
Data given in figure 6.10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





