For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for
Question:
For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for the overall reaction.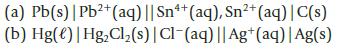
Transcribed Image Text:
(a) Pb(s) | Pb²+ (aq) || Sn4+ (aq), Sn²+ (aq) | C(s) (b) Hg()| Hg₂Cl₂ (s) | Cl-(aq) || Ag+ (aq) | Ag(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a Oxidation HalfReaction Pbs Pb2aq 2e Reduction Hal...View the full answer

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for the overall reaction. (a) Cu(s) | Cu+ (aq) || Fe+ (aq), Fe+ (aq) | Pt(s) (b)...
-
Each of the following equations describes a reaction of a compound called methyl formate. To what class of compounds does methyl formate belong? Which reactions require a reducing agent? Which...
-
Write balanced equations for each of the following reactions (some of these are analogous to reactions shown in the chapter). (a) Aluminum metal reacts with acids to form hydrogen gas. (b) Steam...
-
In Problems 65-72, summarize all pertinent information obtained by applying the graphing strategy, and sketch the graph of y=f(x). 66. 68. 70. 72. x2x6 f(x) 2x 1x +14 f(x)-_-x2-4 x3-5x2-6x 3x +2...
-
Prepare an income statement through gross profit for Brewster Company using the variance data in Practice Exercises 23-1A, 23-2A, 23-3A, and 23-4A. Assume Brewster sold 1,500 units at $80 per unit.
-
Refer to Sport Ready in E7- 19A. If the company can decrease its variable costs to $ 0.80 per package by increasing its fixed costs to $ 105,000, how many packages will it have to sell to generate $...
-
Briefly explain the importance of integrating a recognition strategy as part of known management processes. AppendixLO1
-
Dutch Truck Sales sells semitrailers. The current inventory includes the following five semitrailers (identical except for paint color) along with purchase dates and costs: On May 20, 2011, a...
-
Superior Company provided the following data for the year ended December 31 (all raw materials are used in production as direct materials) Selling expenses Purchases of raw materials Direct labor...
-
Use cell notation to depict an electrochemical cell based upon the following reaction that is product-favored at equilibrium. Cu(s) + Cl(g) 2 Cl(aq) + Cu+ (aq)
-
Once you have separated the three salts in Study Question 111 into three test tubes, you now need to confirm their presence. (a) For Pb 2+ ion, one way to do this is to treat a precipitate of PbCl 2...
-
The propagation speed of small-amplitude surface waves in a region of uniform depth is given by \[c^{2}=\left(\frac{2 \pi \sigma}{\lambda ho}+\frac{g \lambda}{2 \pi} ight) \tanh \frac{2 \pi...
-
50 21 2. Determine the inclination and period of the satellite which produced the ground trace below. Show all calculations. Suteite 17 11-140-130-120-110 tonn an 20 6058 am 50 210 0 10 20 30 50 60...
-
This activity aims to provide practical experience in preparing tax forms related to business income and depreciation. It emphasizes the importance of accurate reporting and adherence to tax...
-
How do intersectionality and identity salience intersect within the framework of diversity and inclusion initiatives, and what strategies can organizations employ to address these complexities ?
-
(a) -2-3 3. Evaluate the following determinants: 3 5 (b) |- 58 -8 -2 4 312 4 3 0 (c) 2 245 (d) 3 1 2 245 5 -1 -4
-
The following information pertains to the inventory of Parvin Company for Year 3: January 1 April 1 October 1 Beginning inventory 400 units @ $22 Purchased 2,600 units @ $27 Purchased 1,200 units @...
-
Let A and B be m x n matrices. (a) Suppose that vTAw = vT B w for all vectors v, w. Prove that A = B. (b) Give an example of two matrices such that vT A v = vT B v for all vectors v, but A B.
-
Draw a Feynman diagram for the reaction n + v p + .
-
A bullet of mass 10 g leaves the barrel of a rifle at 300 m/s. Assuming the force on the bullet is constant while it is in the barrel, find the magnitude of this force.
-
What is the approximate speed at which the force of air drag on a car is equal to the weight of the car?
-
A mass with M = 102 kg is attached to the bottom of a block-and-tackle pulley system as depicted in Figure P3.65. How much tension force is needed to keep the mass at its current position? Figure...
-
The company Omega has the following information at the end of the reporting period: Payments to acquire new fixed assets in the amount of 239 700 Depreciation expense for the reporting period...
-
Kenworth Company uses a job-order costing system. Only three jobs-Job 105, Job 106, and Job 107-were worked on during November and December. Job 105 was completed on December 10; the other two jobs...
-
bottem-up sales and top-down sales calculations for: product:- smart toilet Anually sold units:- 370 price per unit:- $3000 price before margin:- $2700 invested capital:- $160000 please provide an...

Study smarter with the SolutionInn App


