For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for
Question:
For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for the overall reaction.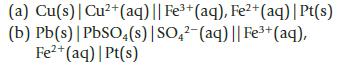
Transcribed Image Text:
(a) Cu(s) | Cu²+ (aq) || Fe³+ (aq), Fe²+ (aq) | Pt(s) (b) Pb(s) | PbSO4(s) | SO4²-(aq) || Fe³+ (aq), Fe²+ (aq) | Pt(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a Cus Cuaq Feaq Feaq Pts Oxidation HalfReaction at the anode Cus Cuaq 2e ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for the overall reaction. (a) Pb(s) | Pb+ (aq) || Sn4+ (aq), Sn+ (aq) | C(s) (b)...
-
Each of the following equations describes a reaction of a compound called methyl formate. To what class of compounds does methyl formate belong? Which reactions require a reducing agent? Which...
-
Write balanced equations for each of the following reactions (some of these are analogous to reactions shown in the chapter). (a) Aluminum metal reacts with acids to form hydrogen gas. (b) Steam...
-
The quarterly sales for a software product over the past three years are given in the table below. 1) Forecast the demand for year 4 using the moving average technique for 3 periods. 1) Compute the...
-
ERP software programs allow tighter linkages within a supply chain than were possible with earlier generations of software. Consider the possibility of a tighter link between the marketing and...
-
Could rent seeking on the part of the CEO influence the CEOs compensation? Evaluate the following argument.
-
Using the Internet, locate the case of a recent lawsuit pitting a franchisor against his or her franchise company. Discuss the merits of the lawsuit. (Hint: Try www.findlaw.com.) AppendixLO1
-
1. Calculate the internal growth rate and sustainable growth rate for S&S Air. What do these numbers mean? 2. S&S Air is planning for a growth rate of 12 percent next year. Calculate the EFN for the...
-
New Age Electronics manufactures surround sound systems and allocates overhead costs using direct-labor hours. They pay their assembly line workers $15 per hour. Unadjusted Cost of Goods Sold for the...
-
In Chapter 13, you learned that entropy, as well as enthalpy, plays a role in the solution process. If H for the solution process is zero, explain how the process can be driven by entropy.
-
Chloroacetic acid, ClCH 2 CO 2 H, is a moderately weak acid (K a = 1.40 10 3 ). If you dissolve 94.5 mg of the acid in water to give 125 mL of solution, what is the pH of the solution?
-
Discuss the relationship between mobile devices and social networking.
-
Statistics Assignments Using Excel Assignment #4: Measures of Variability Part I Below are ACT composite scores from 20 randomly selected college students. 15 33 20 25 21 24 17 16 20 25 26 21 21 17...
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob ODonnell, a local merchant, to contribute the capital to form a partnership....
-
One of the main purposes of evaluation research is: a. reexamining previously collected data. b. monitoring and improving programs. c. generating rich descriptions of individual perspectives. d....
-
10.13 Sweetlip Ltd and Warehou Ltd are two family-owned flax-producing companies in New Zealand. Sweetlip Ltd is owned by the Wood family and the Bradbury family owns Warehou Ltd. The Wood family has...
-
distribution that is skewed to the right instead of being normally distributed. Assume that we collect a random sample of annual incomes of 50 statistics students. Can the distribution of incomes in...
-
The subspace of R3 spanned by the vectors and u2 = (0, 1, 0) is a plane passing through the origin. Express w = (1, 2, 3) in the form w = w1 + w2, where w1 lies in the plane and w2 is perpendicular...
-
Subprime loans have higher loss rates than many other types of loans. Explain why lenders offer subprime loans. Describe the characteristics of the typical borrower in a subprime consumer loan.
-
For laser action to occur, the medium used must have at least three energy levels. What must the nature of each of these levels be?
-
If Plancks constant were smaller than it is, would quantum phenomena be more or less conspicuous than they are now?
-
In the Bohr model of the hydrogen atom, the radius of the electrons orbit in the ground state is 5.3 10 -11 m. What aspect of the quantum-mechanical model of this atom would you expect to correspond...
-
E 5-9 Present value; annuities L05-8 Using the appropriate present value table and assuming a 12% annual interest rate, determine the present value on December 31, 2021, of a five-period annual...
-
QUESTION 16 One of your tax clients has asked you a question about terminating his partnership interest. All of the following may result in the termination of a partnership interest, except :...
-
Credit Debit $ 8,000 17,500 3,000 172,000 $ 36,000 85,000 Account Title Cash Accounts receivable office supplies Trucks Accumulated depreciation-Trucks Land Accounts payable Interest payable...

Study smarter with the SolutionInn App


