If an electrolytic cell for producing F 2 (Figure 21.34) operates at 5.00 10 3 A
Question:
If an electrolytic cell for producing F2 (Figure 21.34) operates at 5.00 × 103 A (at 10.0 V), what mass of F2 can be produced per 24-hour day? Assume the conversion of F− to F2 is 100%.
Data given in Figure 21.34
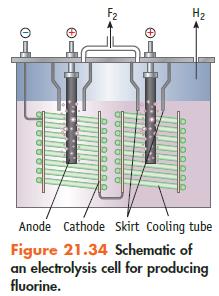
Transcribed Image Text:
H₂ Anode Cathode Skirt Cooling tube Figure 21.34 Schematic of an electrolysis cell for producing fluorine.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
According to Faradays law of electrolysis W Ite ...View the full answer

Answered By

Somil Agarwal
I am a student of NISER, BBSR which is the biggest research institute in India after IISc, Banglore.
I am a theoretical Physicist specialized in Condensed Matter.
I know Quantum Mechanics, Many-body Physics, Electronics, Solid State Physics, Classical Mechanics, Numerical Analysis, Linear Algebra, Probability Theory, Differential Equation, Quantum Information and many other topics related to Physics and Mathematics.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
What mass of AgI can be produced from a 0.512-g sample that assays 20.1% AlI3?
-
Elemental calcium is produced by the electrolysis of molten CaCl2. (a) What mass of calcium can be produced by this process if a current of 7.5 103 A is applied for 48 h? Assume that the...
-
The city of Opelika was having a problem ting land for a new sanitary landfill when the Alabama Energy Extension Service offered the solution of burning the solid waste to generate steam. At the same...
-
A machine was sold in December 20x3 for $13,000. It was purchased in January 20x1 for $19,000, and depreciation of $16,000 was recorded from the date of purchase through the date of disposal....
-
First Security Bancorp Inc. wishes to evaluate three capital investment projects by using the net present value method. Relevant data related to the projects are summarized as follows: Instructions...
-
Describe common audit procedures to audit dividends and retained earnings.
-
Check that the determinant of diagonal and triangular matrices is the product of elements on the diagonal.
-
Melissa Crupp is the new manager of the materials storeroom for Canton Manufacturing. Melissa has been asked to estimate future monthly purchase costs for part #4599, used in two of Canton's...
-
What assertions when the sales.2 transactions have been recorded in the .proper period a) Completeness b) Accuracy c) Classification d) Cutoff e) Existence 2) (2 ) b do e
-
Halogens combine with one another to produce interhalogens such as BrF 3 . Sketch a possible molecular structure for this molecule, and decide if the FBrF bond angles will be less than or greater...
-
To prepare chlorine from chloride ion a strong oxidizing agent is required. The dichromate ion, Cr 2 O 7 2 , is one example (Figure 21.35). Consult the table of standard reduction potentials...
-
Fowler and Christakis (2008) report that personal happiness tends to be associated with having a social network including many other happy friends. To test this claim, a researcher obtains a sample...
-
5. [-/0 Points] DETAILS OSPRECALC1 2.2.106. Use algebra to find the point at which the line f(x) = -x 258 -X+ intersects the line h(x) = x+ 91 + 25 10 (x, y) = Additional Materiale MY N
-
What does the graph tells? from your own understanding. CoursHeroTranscribedText 136 DIVIDED ATTENTION COUNTED TIME BACKWARDS 134 1 2 3 130 136 UNDIVIDED ATTENTION COUNTED TIME BACKWARDS 134 5 132...
-
(ii) State Wilkie's updating equation in respect of the force of inflation and explain carefully what each of the components of the equation represents. State also which type of time series process...
-
Compute the double integral D x y dA over the domain D indicated as 0 x 5, x y 2x + 3. (Use symbolic notation and fractions where needed.) f(x, y) A = D
-
4. (10 points) A researcher believes that length of time spent listening to classical music increases memory for previously learned material. She has 4 groups of 5 subjects listen to either 10 min.,...
-
Find the eigenvalues of the matrix C1 C2. Cn A= C1 2 Cn
-
The overall reaction and equilibrium constant value for a hydrogenoxygen fuel cell at 298 K is 2H 2 (g) + O 2 (g) 2H 2 O(l) K = 1.28 10 83 a. Calculate E cell and G 8 at 298 K for the fuel cell...
-
Considering the lens in Problem 6.29, determine its focal length and the location of the focal points with respect to its vertices V 1 and V 2 . Data from Prob. 6.29 Figure P.6.29 shows two identical...
-
Figure P.6.29 shows two identical concave spherical mirrors forming a so-called confocal cavity. Show, without first specifying the value of d, that after traversing the cavity two times the system...
-
Starting with the exact expression given by Eq. (5.5), show that Eq. (6.46) results, rather than Eq. (5.8), when the approximations for 0 and i are improved a bit. 1 ( n2Si R l; NSo ) (5.5) li lo
-
can i know the calculations and citations?
-
WHAT IS PARTNERSHIP? CHARACTERISTICS ADMISSION, RETIREMENT , DEATH OF A PARTNER, DISSOLUTION OF PARTNERSHIP ADVANTAGES DISADVANTAGES
-
1 According to the author of the article, "Why are Increasing Numbers of Women Choosing to be Single?" the term spinsterhood was historically associated with : O Lower-status and less freedom O Legal...

Study smarter with the SolutionInn App


