In Example 4.2, you found that a particular mixture of CO and H 2 could produce 407
Question:
In Example 4.2, you found that a particular mixture of CO and H2 could produce 407 g CH3OH.
![]()
If only 332 g of CH3OH is actually produced, what is the percent yield of the compound?
Data given in Example 4.2
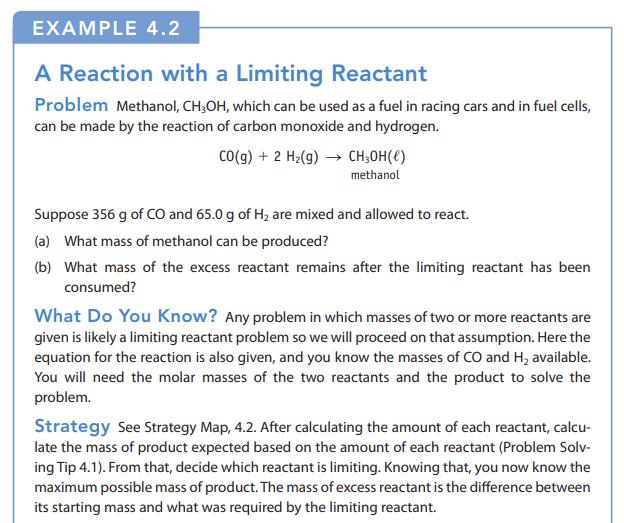
Transcribed Image Text:
CO(g) + 2 H₂(g) → CH₂OH()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To calculate the percent yield of CH3OH we can use the following equatio...View the full answer

Answered By

Muhammad Khurram
I have strong General Management skills to apply in your projects. Over last 3 years, I have acquired great knowledge of Accounting, Auditing, Microsoft Excel, Microsoft PowerPoint, Finance, Microsoft Project, Taxation, Strategic Management, Human Resource, Financial Planning, Business Planning, Microsoft Word, International Business, Entrepreneurship, General Management, Business Mathematics, Advertising, Marketing, Supply Chain, and E-commerce. I can guarantee professional services with accuracy.
4.80+
249+ Reviews
407+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
5. For the circuit in Figure below, use Thevenin's theorem to find the output voltage vo 3i in 2 H 492 www 292 12 cos(1) V F= 118 HH
-
You decide to help I. M. with his analysis. A good fax machine will cost $500 and functions properly for five years. The phone company charges $300 for installing a new line and $60 a month for the...
-
The ABC marketing consulting firm found that a particular brand of tablet PCs has the following demand curve for a certain region: Q = 10,000 - 200P + 0.03Pop + 0.6I + 0.2A where Q is the quantity...
-
You have just been given a $454,000, which you decide to invest at an APR of 6.7 percent. If you were to withdraw $38,500 at the end of each year, starting at the end of this year, how many years...
-
Mop and Broom Manufacturing estimates that it takes 4.5 hours for each broom to be produced, from raw materials to final product. An evaluation of the process reveals that the amount of time spent...
-
Derive an expression for the magnitude of the couple M so that the linkage is in equilibrium for the position shown. To
-
Building an Income Statement The UK insurance firm, Wheeler & Fox, had revenue of 38,440 million, total expenses of 37,133 million, tax of 487 million and zero depreciation. What is the net income...
-
Smithston Corporation leased equipment to Dayplanner Co. on January 1, 2011. The terms of the lease called for annual lease payments to be made at the first of each year. Smithstons implicit interest...
-
Sharp Company manufactures a product for which the following standards have been set: Direct materials Direct labor Standard Quantity Standard Price Standard or Hours or Rate Cost 3 feet $5 per foot...
-
Aspirin, C 6 H 4 (OCOCH 3 )CO 2 H, is produced by the reaction of salicylic acid, C 6 H 4 (OH)CO 2 H, and acetic anhydride, (CH 3 CO) 2 O . If you mix 100. g of each of the reactants, what is the...
-
In the thermite reaction, iron(III) oxide is reduced by aluminum to give molten iron. If you begin with 10.0 g of Fe 2 O 3 and 20.0 g of Al, (a) Which reactant is limiting? (b) What mass of Fe can be...
-
Roberto Gonzalez wants to make his solutions more creative. When he has a problem to solve, he sits down at his desk and tries to generate as many alternative solutions as he can. Unfortunately, he...
-
speed of the three phase motor does not vary greatly from the experiment. 1. Draw the symbol for a Three Phase Electric Motor. (Hint: remember the symbol table from the beginning of the semester?) 2....
-
Lifetime Insurance Company has two supporting departments (actuarial and premium), and two production departments (advertising and sales). Data from operations for the current year are as follows:...
-
Consider a wireless local area network (LAN) with an access point and 10 stations (Station 1, Station 2, Station 3, , and Station 10). Distributed coordination function (DCF), which is based on...
-
A worker needs to pump water from a reservoir to a big container that is open to the atmosphere. The water velocity at the surface of the reservoir is 2.5 m/s. The worker uses a 35-m long, 18-cm...
-
Identify each fringe benefit provided to Maggie and determine whether an exemption applies. (6 marks) Question 2: Explain the impact the fringe benefits will have on Maggie's taxable income and/or...
-
Access the financial statements and related disclosure notes of Ford Motor Company from its website at corporate.ford.com. In Ford's balance sheet, deferred income taxes in 2013 are reported as both...
-
1. Below is depicted a graph G constructed by joining two opposite vertices of C12. Some authors call this a "theta graph" because it resembles the Greek letter 0. a. What is the total degree of this...
-
Each of the following syntheses will not produce the desired product. In each case, identify the flaw in the synthesis. (a) (b) (c) (d) NO2 1) HNO3, H2SO, 2) EECI, AICI3 Et Br 1) Br2, FeBrz 2) AICI,
-
When para-bromotoluene is treated with sodium amide, two products are obtained. Draw both products, and propose a plausible mechanism for their formation.
-
Picric acid is a military explosive formed via the nitration of phenol under conditions that install three nitro groups. Draw the structure and provide an IUPAC name for picric acid.
-
Both employees and employers have to worry about tax in Oman, including social security contributions. Employers must contribute 10.5% of wages to social security and another 1% for industrial...
-
Straight-line depreciation on equipment is an example of a mixed cost O fixed cost O variable cost O step cost
-
Under what circumstances can a financial statement user KNOW that a company is going forward if there is a DECREASE in year-over-year retained earnings

Study smarter with the SolutionInn App


