Mercury vapor is dangerous because breathing it brings this toxic element into the lungs. We wish to
Question:
Mercury vapor is dangerous because breathing it brings this toxic element into the lungs. We wish to estimate the vapor pressure of mercury at two different temperatures from the following data: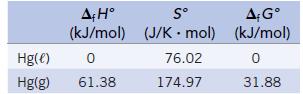
Estimate the temperature at which Kp for the process Hg(ℓ) ⇄ Hg(g) is equal to 1.00 (and the vapor pressure of Hg is 1.00 bar). Next, estimate the temperature at which the vapor pressure is (1/760) bar. (Experimental vapor pressures are 1.00 mm Hg at 126.2°C and 1.00 bar at 356.6°C.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





